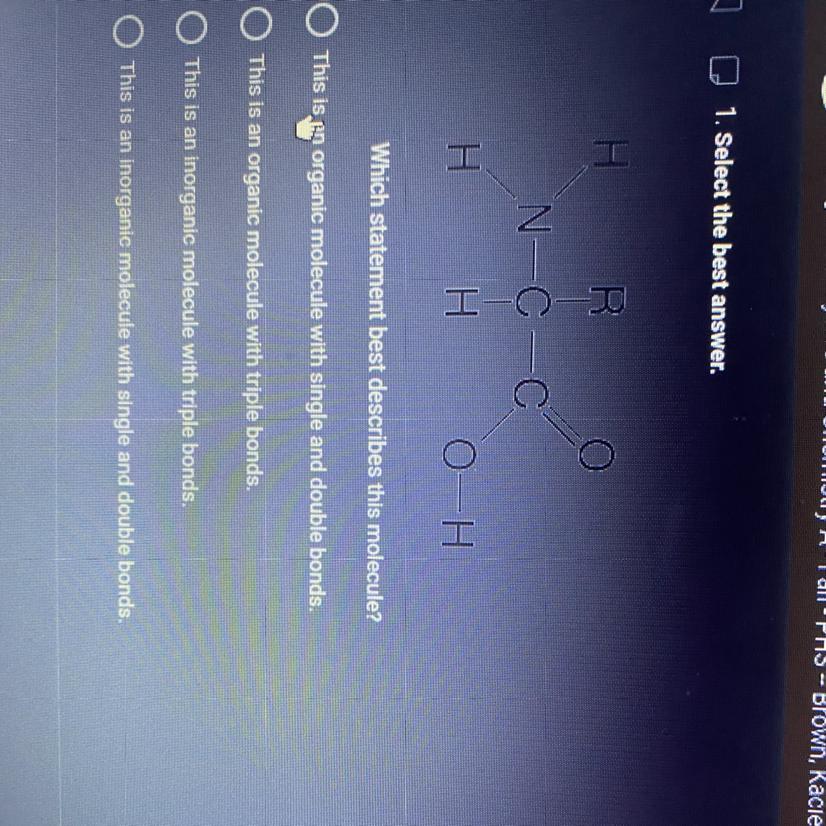
Answers
Answer:
A
Explanation:
The molecule is an amino acid which is used to make proteins, so it is an organic molecule.
It has a double bond between c=o
and single bonds throughout the molecule
Hope this helps!
Related Questions
Examine the table According to the table, which reaction is correct.
Answers
Answer:
Third option, because copper (Cu) is a stronger oxidizing agent than zinc (Zn).
Explanation:
The table shows the Oxidation Half-Reaction, so the elements oxidizes.
When an element is oxidized, it causes the other element in the reaction to be reduced, therefore the element being oxidized is a reducing agent.
In this case, the correct reaction is:
[tex]Zn+Cu^{2+}\rightarrow Zn^{2+}+Cu[/tex]because, copper (Cu) is a stronger oxidizing agent than zinc (Zn).
when is standard reduction potential decreasing
Answers
In general, the ions of very late transition metals those towards the right-hand end of the transition metal block, such as copper, silver and gold have high reduction potentials. In other words, their ions are easily reduced.
Explanation:Again, note that when calculating E∘cell E cell ∘ , standard reduction potentials always remain the same even when a half-reaction is multiplied by a factor. Standard electrode potential - When the concentration of all the species is taken as unity in the half-cell reaction, then the calculated electrode potential is called the standard electrode potential. The reduction potential of a species is its tendency to gain electrons and get reduced. It is measured in millivolts or volts. Larger positive values of reduction potential are indicative of a greater tendency to get reduced. The electrode potential depends upon the concentrations of the substances, the temperature, and the pressure in the case of a gas electrode.
Suppose 3.04 g of copper is reacted with excess nitric acid according to the given equation. Cu(s) + 4NHO3(aq) ---> Cu(NO3)2(aq) + 2NO2(g) + 2H2O(1)
What is the theoretical yield of Cu (NO3)2?
Answers
Taking into account the reaction stoichiometry, the theoretical yield of Cu(NO₃)₂ is 8.9717 grams.
Reaction stoichiometryIn first place, the balanced reaction is:
Cu + 4 NHO₃ → Cu(NO₃)₂ + 2 NO₂ + 2 H₂O
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
Cu: 1 moleNHO₃. 4 molesCu(NO₃)₂: 1 moleNO₂: 2 molesH₂O: 2 molesThe molar mass of the compounds is:
Cu: 63.55 g/moleNHO₃: 63 g/moleCu(NO₃)₂: 187.55 g/moleNO₂: 62 g/moleH₂O: 18 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
Cu: 1 mole ×63.55 g/mole= 63.55 gramsNHO₃: 4 moles ×63 g/mole= 252 gramsCu(NO₃)₂: 1 mole ×187.55 g/mole= 187.55 gramsNO₂: 2 moles ×62 g/mole= 124 gramsH₂O: 2 moles ×18 g/mole= 36 gramsTheoretical yieldThe theoretical yield is the amount of product acquired through the complete conversion of all reagents in the final product, that is, it is the maximum amount of product that could be formed from the given amounts of reagents.
Theoretical yield of Cu(NO₃)₂The following rule of three can be applied: if by reaction stoichiometry 63.55 grams of Cu form 187.55 grams of Cu(NO₃)₂, 3.04 grams of Cu form how much mass of Cu(NO₃)₂?
mass of Cu(NO₃)₂= (3.04 grams of Cu× 187.55 grams of Cu(NO₃)₂)÷ 63.55 grams of Cu
mass of Cu(NO₃)₂= 8.9717 grams
Finally, the maximum amount of Cu(NO₃)₂ is 8.9717 grams.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
brainly.com/question/24653699
#SPJ1
A newly discovered element, X, has been found to have the isotopes listed below (with masses and % abundances). Calculate the average atomic mass.
ISOTOPE
MASS (amu)
%
ABUNDANCE
15.0
21.0
64.0
71x
75x
77x
71.007
74.996
77.112
Type your answer.....
Answers
Average atomic mass of newly discovered element X is 166
What is average atomic mass?
It is calculated by adding the masses of the element's isotopes each multiplied by its natural abundance on Earth
Given data
Isotope Mass % abundance
15 71 71.007
21 75 74.996
64 77 77.112
Contribution made by each isotope by multiplying natural abundance with mass
Average atomic mass
= (71 × 71.007 + 75 × 74.996 + 77 × 77.112) / 100
= 166
Therefore average atomic mass is 166
Learn more about Average atomic masses at https://brainly.com/question/24666479
#SPJ13
Determine the volume of hydrogen gas needed to react completely with 6.00 L of oxygen gas to form water. (20pts)2H2(g) + O2(g) ⇨ 2H2O(g) at STP
Answers
Explanation
Given
2H2(g) + O2(g) ⇨ 2H2O(g)
Volume = 6.00 L
At STP: Temperature = 273K
Pressure = 1 atm
We know that R constant = 0.0821 L.atm/mol.K
Solution
Step 1: Calculate the number of moles of oxygen at STP
Use the ideal gas law equation:
PV = nRT where P is the pressure, V is the volume, n is the number of moles, R is the gas constant and T is the temperature.
n = PV/RT
n = (1 atm x 6.00 L)/(0.0821 L.atm/mol.K x 273 K)
n = 0.27 mol
Step 2: Use the stoichiometry to find the moles of H2
The molar ratio between oxygen and hydrogen is 1:2
Therefore the moles of H2 = 0.27 x (2/1) = 0.535 mol
Step 3: Calculate the volume of H2
V = nRT/P
V = (0.535 mol x 0.0821 L.atm/mol.K x 273 K)/1 atm
V = 12.0 L
Answer
Volume of hydrogen = 12.0 L
Consider the following redox reaction: 2Ag+(aq) + Zn(s) --> Zn^2+ (s) + 2Ag(s)Which of the following statements are TRUE? (Select All That Apply)a) Ag+ is the oxidizing agentb) Ag+ is reduced to Agc) Zn oxidize to Zn^2+d) Zn is the reducing agente) Zn reduce to Zn^2+d) Ag+ oxidize to Zn^2+
Answers
ANSWER
Ag+ is the oxidizing agent
Ag+ is reduced to Ag
Zn is oxidized to zn^2+
Zn is the reducing agent
option ABCD
STEP-BY-STEP EXPLANATION:
Given information
[tex]2Ag^+_{(aq)}+Zn_{(s)\text{ }\rightarrow\text{ }}Zn^{2+}_{(s)}+2Ag_{(s)}[/tex]From the above redox reaction, you will see that there is a change in the oxidation number of zinc and silver.
Zn goes from 0 to 2+ in its oxidation state
Hence, zinc has been oxidized, therefore, it is a reducing agent
Ag goes from +1 to 0 in its oxidation state
Hence, Ag has been reduced, therefore, it is an oxidizing agent
2. The same ratios can be used to predict the mass of the products given the mass of the reactants.a. A sample of copper (II) sulfate pentahydrate weighs 5.9 grams.How many grams of water would be released if all of the water is removed from the pentahydrate?Pls see pictures for details
Answers
Answer
2.12872 grams of water
Explanation
Given:
Mass of copper (II) sulfate pentahydrate sample = 5.9 grams
What to find:
The grams of water that would be released if all of the water is removed from the 5.9 grams sample of copper (II) sulfate pentahydrate.
Step-by-step solution:
The chemical formula of copper (II) sulfate pentahydrate is: CuSO₄.5H₂O
Molar mass of CuSO₄.5H₂O = 249.68 g/mol
Mass of water in 1 mole of CuSO₄.5H₂O = 5 x molar mass of water = 5 x 18.01528 g/mol = 90.0764 g/mol
the percentage by mass of water in 1 mole of CuSO₄.5H₂O is
[tex]\begin{gathered} \%\text{ }by\text{ }mass\text{ }of\text{ }water=\frac{Mass\text{ }of\text{ }water}{Molar\text{ }mass\text{ }of\text{ }CuSO₄.5H₂O}\times100\% \\ \\ \%\text{ }by\text{ }mass\text{ }of\text{ }water=\frac{90.0764}{249.68}\times100\% \\ \\ \%\text{ }by\text{ }mass\text{ }of\text{ }water=36.08\% \end{gathered}[/tex]Hence, you can now use the % by mass of water in 1 mole of copper (II) sulfate pentahydrate to determine the mass of water that would be released in 5.9 grams of copper (II) sulfate pentahydrate as shown below.
[tex]\begin{gathered} Mass\text{ }of\text{ }water=\frac{36.08}{100}\times5.9\text{ }grams \\ \\ Mass\text{ }of\text{ }water=0.3608\times5.9grams \\ \\ Mass\text{ }of\text{ }water=2.12872\text{ }grams \end{gathered}[/tex]Therefore, the grams of water that would be released if all of the water is removed from the 5.9 grams of copper (II) sulfate pentahydrate is 2.12872 grams
Calculate the [OH−] of each aqueous solution with the following [H3O+]:blood, 4.5×10−8M
Answers
Answer
2.22 x 10⁻⁷ M
Procedure
To calculate the [OH⁻] concentration of blood we will consider the following pH formulas
pH=-log [H₃O⁺]
pH+pOH=14
The pH of blood will be
[tex]pH=-\log_{10}[4.5\times10^{-8}M]=7.3467[/tex]Then we do the rest
[tex]pOH=14-7.3467=6.6532[/tex]Lastly, we transform from pOH to concentration
[tex][OH^-]=10^{-pOH}=10^{-6.6532}=2.22\times10^{-7}[/tex]Please help me figure out how to do this question
Answers
Given that molar molar enthalpy of combustion of cyclohexanone is -3468.1 kJ/mol when liquid water is formed, what is the molar enthalpy of formation of cyclohexanone?
What is the percent by mass concentration of salt in a solution made by dissolving 3.5 g of salt in 75 kg of water?Please include equation used and variable you solved for.
Answers
Answer:
Explanations:
The formula for calculating the percentage by mass of concentration is expressed as;
[tex]undefined[/tex]A 45.0 ml sample of nitric acid is neutralized by 119.4 ml of 0.2M NaOH. What is the molarity of the nitric acid?
Answers
0.53L
Explanations:According to dilution formula:
[tex]C_1V_1=C_2V_2[/tex]C1 and C2 are the initial and final concentrations
V1 and V2 is initial and final volume
Given the following parameters
• V1 = 45mL
,• C2 = 0.2M
,• V2 = 119.4mL
Substitute the given parameters into the formula
[tex]\begin{gathered} C_1=\frac{C_2V_2}{V_1} \\ C_1=\frac{0.2\times119.4}{45} \\ C_1=\frac{23.88}{45} \\ C_1=0.53M \end{gathered}[/tex]Hence the molarity of the nitric acid is 0.53L
Describe the bonding in aluminium
Answers
• +Aluminium forms a non- directional metalic bond.
• (bonding in chemistry is the lasting attraction between atoms , ions or molecule )... think of it like joining together of particles / elements
,• This type of covalent bonding results in a metallic lattice structure.
• Since aluminium is a metallic solid, the maximum covalency of aluminium is three only.
See the drawing below:
12. Which two elements have chemical properties that are most
similar?
A.C and N
B. Cl and Ar
C.K and Ca
D.Li and Na
Answers
Li and Na two elements have chemical properties that are most similar
The element that have the most similar chemical properties are those in the same group or column of the periodic table and one such group includes lithium sodium potassium these element are all shiny and conduct heat and electricity well and have similar chemical properties and the element in a group have the same number of electron in the outermost shell hence they have similar chemical properties
Know more about element
https://brainly.com/question/491273
#SPJ1
In a combustion reaction, acetylene (C2H2) combines with oxygen gas to form carbon dioxide and water. Write and balance the equation for this reaction.Help me please
Answers
A complete combustion reaction like the one you mention is a reaction that will produce carbon dioxide and water using oxygen as the oxidizing agent. The fuel can vary and from there different numbers of CO2 and water will be obtained. Let us first write what the reaction would be taking into account the unbalanced reactants and products:
[tex]C_2H_2+O_2\rightarrow CO_2+H_2O[/tex]Now, to balance the equation we must count the number of atoms of each element on each side of the reaction.
In the previous scheme, we see that neither the carbon nor the oxygen are balanced, we can start by balancing the carbon. We have two carbon atoms in the reactants, so we put the coefficient two in front of the CO2 molecule.
I will be updating the scheme as I describe the procedure so that you are aware of it.
Now we must balance the oxygen, we have 5 atoms in the products and two in the reactants. To balance it, since we have an odd number in the products, we must adjust it to an even number. We place coefficient 2 in front of the H2O molecule. We have 6 oxygen atoms in the products, to have 6 oxygen atoms in the reactants we must place the coefficient 3 in front of the O2 molecule.
Now we have 6 oxygen atoms on each side of the reaction, we continue now with hydrogen. We have 4 hydrogens in the reactants, so we put the coefficient 2 in front of the C2H2 molecule.
Now the hydrogen is balanced, but the number of carbons changed and we must balance them again. We place the coefficient 4 in the CO2 molecule, so we will have 4 carbons on each side of the reaction.
In doing so, the number of oxygens was modified, so to finally have a balanced reaction we put the coefficient 5 in the O2 molecule to have 10 oxygen atoms on each side.
So, the balanced reaction will be:
[tex]2C_2H_2+5O_2\rightarrow4CO_2+2H_2O[/tex]What is the volume of 0.110 g of C2H2F4 vapor at 0.887 atm and 34.0°C?Answer in units of L.Leave all the numbers after the decimal point (No usage of significant figures)
Answers
Assume that C₂H₂F₄ vapor is an ideal gas and use the ideal gas law to find the volume of the vapor.
First, use the molecular mass of the compound to find the number of moles present in 0.110g of the compound.
[tex]0.110g\cdot\frac{1mol}{102.03g}=0.00108mol[/tex]Convert the temperature in celsius to kelvin:
[tex]K=34+273.15=307.15[/tex]Use the ideal gas law and solve it for V:
[tex]\begin{gathered} P\cdot V=n\cdot R\cdot T \\ V=\frac{n\cdot R\cdot T}{P} \end{gathered}[/tex]Replace for the known values (R, the ideal gas constant is 0.082 atmL/molK):
[tex]\begin{gathered} V=\frac{0.00108mol\cdot0.082\frac{atmL}{molK}\cdot307.15K}{0.887atm} \\ V=0.0306L \end{gathered}[/tex]The volume of the vapor is 0.0306L.
Consider the neutralization reaction2 HNO3(aq) + Ba(OH)₂ (aq) -> 2 H₂O(l) + Ba(NO3)₂(aq)A 0.125 L sample of an unknown HNO, solution required 42.7 mL of 0.100 M Ba(OH)₂ for complete neutralization. What isthe concentration of the HNO, solution?
Answers
Answer:
C = 0.068 M
Explanation:
We are given: volume of HNO = 0.125 L
: volume of Ba(OH)2 = 42.7 mL
: concentration of Ba(OH)2 = 0.100 M
We first determine the number of moles of Ba(OH)2:
n = CV
Where: n is number of moles, C is concentration, and V is volume
n = CV
= 0.0427 * 0.100
= 0.00427 mol
From the balanced equation:
n(HNO3) = 2 * n(Ba(OH)2)
= 2 * 0.00427
= 0.00854
For HNO3:
C = n/V
= 0.00854/0.125
= 0.068 M
Which of the following statements is true of the internal energy of a system and its surroundings during an energy exchange with a negative ∆Esys?
a. The internal energy of the system and the surroundings increases
b. The internal energy of the system and the surroundings decreases.
c. The internal energy of the system increases and the surroundings decreases.
d. The internal energy of the system decreases and the surroundings increases.
Answers
The system's internal energy decreases while the environment's internal energy rises (option -D) is correct answer.
What is the definition of a system's internal energy?The internal energy of a system with specific boundaries is composed of the kinetic energy produced by the motion of molecules, the potential energy produced by the vibrational motion, and the electric energy of atoms within molecules. These three types of energy are added together.
The internal energy is equivalent to the system's heat. Since heat is neither created nor destroyed, as the environment's heat level rises, so does the system's heat level.
The gas's state is the only factor that influences its internal energy. In an isothermal process, the volume graph of the change in internal energy in the maximum area under pressure equals the heat supplied in any process. Work is dependent on state but not on a path.
To know more about internal energy of a system visit:
https://brainly.com/question/11742607
#SPJ1
What is the balanced chemical equation for the decomposition of mercury (II) oxide?
Answers
So,
The balanced chemical equation for the decomposition of mercury (II) oxide is:
A lead block, initially at 58.5oC, is submerged into 100.0 g of water at 24.8oC, in an insulated container. The final temperature of the mixture upon reaching equilibrium is 26.2oC. What is the mass of the lead block?
Answers
Answer:
The specific heat capacity of lead is 0.128 J/g°C.
Mass of lead block = (100.0 g)(26.2oC - 24.8oC)(0.128 J/g°C) / (58.5oC - 24.8oC)
Mass of lead block = 5.45 g
You could add HCl(aq) to the solution to precipitate out AgCl(s) . What volume of a 0.100 M HCl(aq) solution is needed to precipitate the silver ions from 16.0 mL of a 0.160 M AgNO3 solution?Express your answer with the appropriate units.
Answers
The volume of a 0.100 M solution needed is 256 mL.
A chemical reaction, the transformation of one or more chemicals (the reactants) into one or more distinct compounds (the products). Chemical elements or chemical compounds make up substances.
From the question,
The chemical reaction is:
AgNO₃ + HCl → AgCl + HNO₃
The volume of silver ions is V = 16 mL = 0.16 L
Concentration of AgNO₃, c( AgNO₃ ) = 0.160 mol/L
Moles of AgNO₃, n( AgNO₃ ) = 0.16L × 0.160 mol/L = 0.0256 mol
From the reaction,
n( AgNO₃ ) : n( HCl ) = 1 : 1
0.0256 : n( HCl ) = 1 : 1
Therefore,
n( HCl ) = 0.0256 mol
The volume of hydrochloric acid is:
V( HCl ) = n( HCl ) ÷ c( HCl )
V( HCl ) = 0.0256 mol ÷ 0.100 mol/L
V( HCl ) = 0.256 L
V( HCl ) = 256 mL
Learn more about volume here:
brainly.com/question/27100414
#SPJ9
how does energy and intermolecular forces affect water evaporation.
Answers
Evaporation is a phenomenon in which atoms of substances in a liquid state obtain enough energy to change to a gaseous state. The movement caused by the temperature of the molecules must be sufficient to overcome the surface tension of the substances and achieve the evaporation process. The molecules must have enough energy to
break intermolecular interactions and go to the gaseous state. Breaking up intermolecular interactions requires energy, which is usually given through heat. Therefore, this process is an endothermic reaction.
Which element is in the same group as Lithium (Li)?
Carbon (C)
Potassium (K)
Chlorine (Cl)
Hydrogen (H)
Answers
Answer: Potassium (K)
Explanation:
9. I have a pressure of 65 kPa and a starting temperature of29°C. If I raise the temperature to 167°C, what is the newpressure?
Answers
Answer:
[tex]94.70\text{ kPa}[/tex]Explanation:
Here, we want to get the new pressure
According to the pressure law, temperature and pressure are directly proportional
Mathematically, we have that as:
[tex]\text{ }\frac{P_1}{T_1}\text{ = }\frac{P_2}{T_2}[/tex]where:
P1 is the initial pressure which is 65 KPa
P2 is the final pressure that we want to calculate
T1 is the initial temperature which we will convert to Kelvin by adding 273K:
We have T1 as 29 + 273 = 302K
T2 is the final temperature which is also 167 + 273 = 440K
Substituting the values, we have it that:
[tex]\begin{gathered} \frac{65}{302}\text{ = }\frac{P_2}{440} \\ \\ P_2\text{ = }\frac{440\times65}{302}\text{ = 94.70 kPa} \end{gathered}[/tex]The combining of hydrogen and oxygen gas into liquid water is an example of which type of change?
Answers
The combining of hydrogen and oxygen gas into liquid water is an example of which type of change physical change.
A physical change occurs when hydrogen and oxygen are combined to create water. Concrete breaking up is a physical change. A chemical change occurs when beach sand would be washed out to sea.
When hydrogen will react with oxygen then it will form water. It is a kind of combination reaction. Its chemical reaction can be expressed as:
H2 + O2 → H2O
Therefore, this reaction is an example of physical change.
To know more about physical change
https://brainly.com/question/17931044
#SPJ1
Percent is the amount of one part of a mixture per 1000 total parts.A)trueB)false
Answers
The word percent is telling us 'percent' is the amount of one part in every hundred (100), so the given statement of the question is false.
Aspirin is a common analgesic. If you want to produce 500. mg of aspirin (C9H8O4, m.w. = 180.16 g/mol) from the reaction of C7H6O3 (m.w. = 130.12 g/mol) and C4H6O3 (m.w. = 102.09 g/mol), what is the minimum amount of C4H6O3 that is needed?2C7H6O3(s) + C4H6O3(l) → 2C9H8O4(s) + H2O(l)
Answers
1) First, let's rewrite the chemical equation here:
2 C7H6O3(s) + C4H6O3(l) → 2 C9H8O4(s) + H2O(l)
2) Let's find out how many moles of C9H8O4 have into 500 mg of it. For this, use ese the following equation:
mole = mass/ molar mass
mass = 500 mg = 0.5 g
molar mass of C9H8O4 = 180.16 g/mol (the question give it to us)
mole = 0.5/180.16
mole = 0.0028 moles of C9H8O4
3) Using the chemical equation proportion, we can discover the amount in moles of C4H6O3 needed to form 0.0028 moles of C9H8O4. So:
1 mol of C4H6O3 forms 2 moles of C9H8O4
x mol of C4H6O3 forms 0.0028 moles of C9H8O4
2x = 0.0028
x = 0.0014 moles of C4H6O3
4) We need to find the answer in grams. Let's transform 0.0014 moles of C4H6O3 into grams. For this, use ese the following equation:
mass = mole × molar mas
mass = 0.0014 moles × 102.09
mass = 0.142 grams or 1.42x10⁻¹
Answer: The amount of C4H6O3 that is needed is 1.42x10⁻¹ g.
can you help me with this, please?which anion will form a precipitate with Na+?
Answers
A precipitate formed in a reaction or solution is when we have a solid salt that is not soluble in the reaction anymore, in our case we are dealing only with the compound formed with Sodium and another anion, so the one that is not soluble in water will be the one that will form a precipitate, therefore a solid salt not dissolvable. Out of the three ionic compounds: Na2CO3, Na2CrO4, NaI2, Sodium chromate (Na2CrO4) will present as the less soluble in water, since chromates in general are not very soluble in water, which is not the case for carbonates and iodides, they will readily dissolve in water, not forming any precipitates
Fermentation is the process by which yeast acts upon a sugar in an oxygen-free environment to produceQuestion 9 options:A) amines.B) carboxylic acids.C) alcohols.D) esters.
Answers
Answer
Alcohol
Explanation
Alcoholic fermentation is a biochemical process in which yeast and some kinds of bacteria convert sugars into ethyl alcohol and carbon dioxide.
veredAnswerQuestion 3Suppose the gas inside a closed, steel cylinder has a pressure of 6.1 atm at2.0 °C. What will be the pressure (atm) of the gas at -83.0 °C? Enter youranswer to 2 decimal places.-710/1 pts4.22 margin of error +/- 0.05
Answers
Answer
4.22 atm
Explanation
Given that:
The initial pressure, P₁ = 6.1 atm
The initial temperature, T₁ = 2.0 °C = (2.0 + 273.15 K) = 275.15 K
The final temperature, T₂ = -83.0 °C = (-83.0 + 273.15 K) = 190.15 K
What to find:
The final pressure, P₂ (atm) of the gas at 83.0 °C.
Step-by-step solution:
The relationship between pressure and temperature of gaseous subtance was decribed by Amonton's gas law.
Amonton's Law states that the pressure of an ideal gas varies directly with the absolute temperature when the volume of the sample is held constant.
Mathematically, we have:
[tex]\frac{P_1}{T_1}=\frac{P_2}{T_2}[/tex]Putting the values of the parameters into the formula, we have:
[tex]\begin{gathered} \frac{6.1\text{ }atm}{275.15\text{ }K}=\frac{P_2}{190.15\text{ }K} \\ \\ P_2=\frac{6.1atm\times190.15K}{275.15K} \\ \\ P_2=4.22\text{ }atm \end{gathered}[/tex]Thus, the final pressure, (atm) of the gas at 83.0 °C is 4.22 atm.
Fill in the chart to identify three parts of Dalton's model of the atom.
Answers
The first part of Dalton's model is that all matter is made of atoms, and these atoms are indivisible.
The second part of this model is that atoms of an element have identical masses and identical propertes.
At last, the third part of Dalton's model is that all compounds are formed by two or more different type of atoms combined.