What type of radiation is emitted when an electron in a hydrogen atom drops from energy level 6 to 2?.
Answers
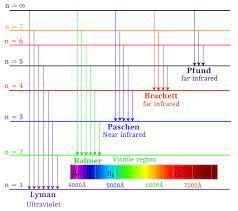
When the electron falls from energy level 6 to energy level 2, it will irradiate Balmer series that lies in the visible region of electromagnetic spectrum.
A hydrogen atom has six spectral lines. Spectral emissions occurs when an electron transitions jumps from a higher energy state to a lower energy state. The spectral lines are grouped into series according to the lower energy level of atomic hydrogen is divided into a number of spectral series, with wavelengths.
Wavelength of these spectral lines can be calculated by the Rydberg formula and these spectral lines are formed due to the electron that undergoes transitions between two energy levels in an atom.
Spectral emissions occurs when an electron transitions jumps from a higher energy level to a lower energy level.
The spectral lines are generally grouped into series according to the lower energy level.
To know more about Hydrogen Spectral:
brainly.com/question/15591186
#SPJ4
Related Questions
Where would one find the s-block, p-block, d-block and f-block elements on the periodic table?
Answers
Answer:
You can find p-block elements on the periodic table in groups 13 to 18, you can find the s-block on the periodic table in group 1, Group 3 through Group 12 are d-block elements, and The f-block elements, found in the two rows at the bottom of the periodic table, are called inner transition metals and have valence electrons in the f-orbital's.
Explanation:
Hope this helps!
which of the following is the only list representing natural components of the atmosphere
Answers
The option containing Pollen, oxygen, carbon dioxide is the correct answer as it contains all the natural components, rest contains one or more synthetic or man made component.
Male gametes or the sperm cells are transported between the different plant reproductive sections by pollen which is a fascinating natural substance that is essential to plant reproduction.
The water is the primary component of all lifeforms, oxygen makes up the majority of the mass of all living things.
There are the numerous natural sources of atmospheric carbon dioxide, including outgassing from the ocean, decomposing vegetation and other biomass, venting volcanoes, naturally occurring wildfires, and even belches from ruminant animals. Photosynthesis is the process by which oxygen is continuously replenished in the Earth's atmosphere.
Pollen, oxygen and carbon dioxide all are natural component since they are form naturally in the environment.
To know more about natural components, please refer:
https://brainly.com/question/4677279
#SPJ1
Your question is incomplete , for full question please refer below:
Which of the following is only list representing natural components of the atmosphere?
Dust, argon, smogPollen, oxygen, carbon dioxide Pollen, sulfuric acid, smogDust, oxygen, sulfuric acidsWhich of the following is true of
the eye of a hurricane?
A. Warm, moist bands of air move around the
eye.
B. The eye makes up the outer most layer of the
storm.
C. Hot and humid air rises up through the eye.
D. It only appears at the very beginning and very
end of the storm.
Answers
Answer: a
Explanation:
what is the molarity of chloride ion in a solution made by dissolving 1.500g of aluminum chloride in a total volume of 250.0ml?
Answers
Answer: The Molarity of chloride ion in the solution is 0.1344 m.
Explanation:
For this question we first have to find the no. of moles of the given compound.
moles of Aluminium chloride is (n)= 1.5/133.5=0.0112. (given mass/molecular mass)
no of chloride ions present in 1 mole of this given compound = 3
moles of chloride ions present in 0.0112 moles of compound = 0.0112x3=0.0336
molarity of CL ion is = 0.0336/(250/1000)=0.1334 m
Learn more about molarity here https://brainly.com/question/12127540
#SPJ4
in the sn1 mechanism conditions, silver nitrate is added. in principle, the nitrate anion can act as a nucleophile, but is so poor of a nucleophile that any chemical reactivity from it is negligible. knowing that, what is the nucleophile in the sn1 reaction in the reactions using silver nitrate if it is not the nitrate ion?
Answers
Nitrate performs an crucial function withinside the technique as nicely with the aid of using performing as a nucleophile that promotes a thermodynamic self-correcting technique.
the response of an alkyl chloride with silver nitrate (AgN03) to shape silver chloride and an alkyl nitrate, the silver atom coordinates with the chloride facilitating the formation of the carbocation. Nitrate ion is one of these susceptible nucleophile, that direct SN2 displacement can't occur.
The nitrate is a terrible nucleophile. It has three oxygen atoms pulling electron density far from the nitrogen, and there may be additionally a truthful quantity of resonance that lowers the nucleophilicity notably as well SN1 response mechanism follows a step-with the aid of using-step technique in which first, the carbocation is shaped from the elimination of the leaving group.
Then the carbocation is attacked with the aid of using the nucleophile. Finally, the deprotonation of the protonated nucleophile takes location to present the specified product.
Learn more about SN1 reaction here https://brainly.com/question/24130879
#SPJ4
3. Categorize the following physical properties as physical properties recognized by our sense organs or measurable physical properties a. Density b. odor c. taste d. melting point e. color f. conductivity
Answers
Melting point, density, color, are measurable physical properties.
A physical property is a characteristic that may be observed or quantified without changing the composition. Density is another illustration of a physical characteristic. Your coin is still composed of the same material as it was before it was dropped into the fountain, despite the fact that it may be a little moist. Additional examples of physical attributes are color, mass, smell, boiling point, volume, and temperature.
Some physical qualities, such density and color, may be detected without changing the physical state of the item being studied. Only when matter undergoes a physical transition can other physical properties, like the melting point of iron or the freezing point of water, be seen.
To know more about physical properties visit : https://brainly.com/question/13562531
#SPJ9
Most of the weather of the world is based upon changes in the moisture, pressure, and/or temperature of the.
Answers
Most of the weather of the world is based upon changes in the moisture, pressure or temperature of the troposphere.
What is the importance of troposphere?The layer closest to the surface of the Earth is troposphere. This layer goes up to twenty kilometers above the Earth. The troposphere is very essential as it contains the weather of the Earth and also greenhouse gases to maintain the temperature on Earth.
Troposphere layer has the air humans breathe and the clouds in the sky. The air is densest in this lowest layer and the troposphere has three-quarters of the mass of the entire atmosphere. The air in troposphere is 78% nitrogen and 21% oxygen.
To know more about troposphere, refer
https://brainly.com/question/818716
#SPJ4
Which of the following describes how difficult it is to remove a valence electron from an atom of a particular element?
A: Electron Affinity
B: Ionization energy
C: Atomic Radius
D: Electronegativity
Answers
Answer:
B: ionization energy
Explanation:
Calculate the volume in liters of a 7.76 g/dL iron(III) bromide solution that contains 427. g of iron(III) bromide (FeBr3).Round your answer to 3 significant digits.
Answers
Answer
5.50 L of solution
Procedure
We will use dimensional analysis to solve this question. After getting the answer, we will round to 3 significant digits. All the previous can be done in a single-step calculation.
[tex]427\text{ g}\frac{1\text{ dL}}{7.76\text{ g}}\frac{1\text{ L}}{10\text{ dL}}=5.5026\approx5.50\text{ L}[/tex]Explain in terms of energy transfer and motion of molecules what happens to the WAX when the candle is blown out.A: When the candle was blown out the wax ________ because the molecules _________ energy and moved _________.
Answers
When a candle is lit, a combustion reaction takes place that releases heat, this heat causes the temperature of the candle to increase and the state of the candle to be liquid. Molecules move due to heat.
When the candle blown out, the temperature begins to decrease and the liquid wax begins to solidify because the molecules move slower and slower. Therefore, the spaces of the obtained can be filled in the following way:
When the candle was blown out the wax solidifies because the molecules have low energy and moved slower.
A student proposed the Bohr model below for sodium (Na). Is this student’s model correct? Justify your answer
Answers
The Bohr model is a representation of the electronic configuration of the atom. According to this model, each energy level can hold a certain number of electrons. In the first energy there can only be 2 electrons, in the second and the following energy levels there can be a maximum of 8 electrons.
Sodium, Na which has 11 electrons in total so in the first level it will have two electrons, in the second level it will have 8 electrons and in the third level it will have the missing electron.
In the model proposed by the student, the electrons are represented in blue. The model proposed by the student is incorrect.
We see that in the second energy level he drew 9 electrons, this is incorrect since from the second energy level there can only be 8 electrons, the remaining electron must be located in the third energy level.
pls answer question will mark brainliset tyty
Answers
Answer: A. The population is growing rapidly
Describe the intermolecular forces in terms of strength and properties?
Answers
Liquids have characteristics that fall somewhere in between those of solids and gases, yet they resemble solids more. Intermolecular forces, as opposed to intramolecular forces, such as the covalent bonds that bind atoms in molecules and polyatomic ions, keep molecules in a liquid or solid together. In comparison to covalent bonds, intermolecular forces are often significantly weaker. For instance, in 1 mol of water, the intramolecular forces must be overcome in order to break both O-H bonds, while the intermolecular forces must be overcome in order to turn 1 mol of liquid water into water vapor at 100 °C, which needs just around 41 kJ. (Despite this figure appearing to be modest, the intermolecular forces in liquid water are some of the highest intermolecular forces ever measured.
What is the compound name of this formula CuSO4 ?
Answers
Copper(II) sulfate.
Explanations:Before we name the given compound, we need to know what chemical compounds are. Chemical compounds are chemical substances composed of molecules formed from atoms of an element and held together by a chemical bond.
Given the chemical compound CuSO4, this compound contains copper, sulfur, and oxygen element.
The molecular compound CuSO4 is named with the first element first and then the second element by using the stem of the element name plus the suffix -ate
The compound name for CuSo4 will therefore be Copper(II) sulfate. The roman numeral of (II) comes from the sum of the oxidation state of the element.
Note that the oxygen atoms have a -2 oxidation number, and sulfur has a +6, oxidation number to have (4 * -2) + 6 = -2. This makes copper have an oxidation number of +2.
what is the total number of electron-pair domains (electron clouds) around the central atom in the sulfur tetrafluoride molecule? enter your answer as a numeral without leading or lagging spaces. g
Answers
Total number of electron-pair domains (electron clouds) around the central atom in the sulfurtetrafluoride molecule is five. which includes 4 bond pair 1 loan pair.
what is electron pair domain?An atom's electron domain is the number of lone pairs or chemical bond locations that surround it. It represents the number of locations expected to contain electrons. By knowing the electron domain of each atom in a molecule, you can predict its geometry.
“The electron geometry describes the spatial arrangement of a molecule’s bonds and lone pairs. VSEPR theory can be used to calculate electron geometry.”
The term electron geometry is the name of the electron pair/groups/domains on the central atom, whether they are bonding electrons or non-bonding electrons. Electron pairs are electrons that exist in pairs or bonds, as lone pairs or as a single unpaired electron. Because electrons are always in motion and their paths cannot be precisely defined, the electron arrangement in a molecule is described in terms of an electron density distribution.
learn more about electron pair domain:https://brainly.com/question/16940491
#SPJ4
Which statement about liquids and gases is correct? A -1 cm³ of gas contains more particles than 1 cm² of liquid.
B- A given mass of liquid has a fixed volume at room temperature.
C- Particles in a liquid can easily be forced closer together.
D Particles in a liquid have fixed positions.
Answers
Answer:
A given mass of liquid has a fixed volume at room temperature.
Explanation:
How many grams of sodium hydroxide (NaOH) do you need to make 6.5 L of 1.0M solution?
Answers
260grams
Explanations:The formula for calculating the moles of NaOH is expressed as
[tex]\begin{gathered} moles=molarity\times volume \\ moles\text{ of NaOH}=1.0M\times6.5L \\ moles\text{ of NaOH}=6.5moles \end{gathered}[/tex]Determine the required mass of NaOH
[tex]\begin{gathered} Mass\text{ of NaOH}=moles\times molar\text{ mass} \\ Mass\text{ of NaOH}=6.5\cancel{moles}\times\frac{40g}{\cancel{mole}} \\ Mass\text{ of NaOH}=260grams \end{gathered}[/tex]Hence the grams of sodium hydroxide (NaOH) you need to make 6.5 L of 1.0M solution is 260grams
The equation below shows hydrogen reacting with oxygen to produce water.
2H2 + O2 Right arrow. 2H2O
If 16 mol of oxygen were reacted with excess hydrogen gas, how many moles of water would be produced?
4.0 mol
8.0 mol
16 mol
32 mol
Answers
The equation below shows hydrogen reacting with oxygen to produce water 2H₂ + O₂ → 2H₂O If 16 mole of oxygen were reacted with excess hydrogen gas, then 32 mole of water would be produced
Mole is the amount of substance of a system which contains as many elementary entities
Here given data is
Hydrogen reacting with oxygen to produce water and the reaction is
2H₂ + O₂ → 2H₂O
16 mol of oxygen were reacted with excess hydrogen gas
Then we have to calculate moles of water would be produced = ?
Moles of oxygen = 16 moles
From the balanced equation
1 mole oxygen react to give 2 moles of water
16 moles oxygen react to give 2×16 = 32 moles
Therefore 32 moles of water would be produced in the reaction
Know more about mole
https://brainly.com/question/10811423
#SPJ1
d. 32 mol in case you dont want to read that
A spray paint can at 24 Celsius has a pressure of 20 psi. If it is placed in a fire at 480 Celsius what will the pressure become?
Answers
According to Gay Lussac's law, the pressure of a given mass of gas is directly proportional to its temperature provided that volume is constant. Mathematically;
[tex]\begin{gathered} P\alpha T \\ P=kT \\ k=\frac{P}{T} \\ k=\frac{P_1}{T_1}=\frac{P_2}{T_2} \end{gathered}[/tex]From the given question, we are given the following parameters:
[tex]\begin{gathered} P_1=20psi \\ T_1=24^0C=24+273=297K \\ T_2=480^0C=480+273=753K \\ P_2=\text{?} \end{gathered}[/tex]Substitute the given parameters into the formula as shown:
[tex]\begin{gathered} P_2=\frac{P_1T_2}{T_1} \\ P_2=\frac{20psi\times753\cancel{K}}{297\cancel{K}} \\ P_2=\frac{15,060}{297} \\ P_2=50.71\text{psi} \end{gathered}[/tex]Therefore the pressure of the spray paint at 480 celsius is 50.71psi
How many electrons can be held in a sublevel l = 3?
Answers
Answer:
6 electrons max
Explanation:
The p sub level has three orbitals so can contain six electrons The d sub levels has five orbitals so can contain 10 electrons Max.
Which formulas represent one ionic compound and one covalent compound?
a. N, and SO.
b. Cl, and H,S
c. BaCl and N.O,
d. Na,O and CaO
Answers
The formula NaO,CaO represents one ionic compound and one covalent compound.
An ionic compound is formed by ions made up of charged particles.A covalent compound is formed by sharing of electrons between two different elements.N exists as N2 which is covalently bonded and SO exists as S=O which is covalently bonded.Cl exists as Cl2 which is formed by sharing of electrons and is covalently bonded.HS has an electronegativity difference of 0.4 which is non-polar covalent compound.BaCl2 exists as an ionic compound as it is formed on basis of electrostatic attraction.NO exists as N=O which is a covalent compound.NaO exists as ionic compound and CaO exists as covalent compound.
To learn more about ionic compound visit:
brainly.com/question/9167977
#SPJ9
What is the molecular formula of the compound?Express your answer as a chemical formula.
Answers
Answer:
[tex]C_2H_4[/tex]Explanation:
Here, we want to get the chemical formula for the compound
From what we have, the compound contains hydrogen and carbon only
We also were given that the molar mass is 28.06 amu
Now,we beed to get the individual atomic mass of carbon and hydrogen
From records, carbon has an AMU of 12.01
The AMU of hydrogen is 1.00794
Looking at the total value, we can see that the max possible number of atoms of carbon present is 2
Now, we need to know the number of hydrogen atoms
Let us call this n
Mathematically, we know that the AMU of the compound is the sum of the AMU of the individual atoms
Hence, we have it that:
[tex]\begin{gathered} 28.06\text{ = 2(12.01) + n(1.00794)} \\ 1.00794n\text{ = 28.06 - 24.02} \\ 1.00794n\text{ = 4.04} \\ \\ n\text{ = }\frac{4.04}{1.00794} \\ \\ n\text{ = 4} \end{gathered}[/tex]Finally, we want to write the molecular formula of the compound
The molecular formula is written with carbon first, followed by its number of atoms as subscript, then hydrogen , with its number of atoms as subscript
We have this written as:
[tex]C_2H_4[/tex]i need help on all of them please & thank you!! Unfortunately Punnett Squares are not always for finding out the best parts about parenting. They are often, as you will see in the next few lessons, often used to determine the probability of a disease being passed on to a child. We’ll have you try one and see what that looks like. One such disease is Cystic Fibrosis. Let’s assume both parents are heterozygous for Cystic Fibrosis. That means both parents carry the recessive gene for the disease, but because of the dominant genes they have, they don’t have it. However, they want to know if they try to have a child, what the chances of the child would be for having Cystic Fibrosis?
Answers
Answer:
question 1:
answered previously
question 2:
a= 50% chance tall daughter
b= 50% chance short daughter
question 3:
a= 75% chance of not having cystic fibrosis
b= 25% chance of having cystic fibrosis
question 3:
Explanation:
question 2:
the question specified on the offspring being female so when stating the gametes we must include the XX chromosomes for the female and XY chromosome for the male.
let us denote that:
T = tall gene
t = short gene
Father 》 Mother
parental phenotype: Short 》 tall
parental genotype: XtYt 》 XTXt
parental gametes: (Xt) (Yt) 》 (XT) (Xt)
random fertilization:
Xt Yt
XT XTXt XTYt
Xt XtXt XtYt
F1 generation sex: Male 》Female
F1 chromosomes: XY 》 XX
F1 gen phenotype: Tall 》 Tall
F1 gen genotype: Tt, tt 》 Tt, tt
ratio: 2 : 2 or 1 : 1
percentage gender: 50% 》 50%
percentage tall: 50% 》 50%
question 3:
denoting:
N = normal (dominant)
c= cystic fibrosis (recessive)
parental phenotype: Normal 》Normal
parental genotype: Nc 》 Nc
random fertilization:
N c
N NN Nc
c Nc cc
F1 gen phenotype: Normal 》 Cystic fibrosis
F1 gen genotype: NN, Nc, Nc 》 cc
ratio: 3 : 1
percentage: 75% 》 25%
(if you have anymore questions on the topic let me know)
Some are based on other
languages, for example the
symbol
is
from the Latin "ferrium."
Answers
Answer:
Fe
Iron – Ferrum (Fe)
Iron's Latin name, 'ferrum', gives it its symbol Fe; it simply means 'iron' or 'sword', and is possibly of Semitic origin.
What is the empirical formula of a compound composed of 81. 71% c and 18. 29% h by mass?.
Answers
m
C
=
0.8171
×
100
g
=
81.71
g
m
H
=
0.1829
×
100
g
=
18.29
g
Using the known molar masses, the molar quantities of carbon and hydrogen in this sample are:
n
C
=
81.71
g
×
m
o
l
12.01
g
=
6.803
m
o
l
n
H
=
18.29
g
×
m
o
l
1.01
g
=
18.11
m
o
l
The molar ratio of the elements is:
C
6.803
H
18.11
If we divide both subscripts by the smallest value and multiply by 3, then the empirical formula will be:
C
3
H
8
calculate how many grams are in 0.10 moles of OH ions
Answers
1.7 grams are there in 0.10 moles of OH ions.
The mole is a unit of measurement or the starting point for determining the concentration of a chemical in a sample. Avogadro's constant, or 1 mole, is a quantity that is equal to 6.022 × 10²³ particles. Any species, such as atoms, molecules, electrons, protons, neutrons, etc., can make up.
Number of moles = Mass/ Molecular mass
Given, number of moles = 0.10
Molecular mass of OH = 17 g/mol
∴ Mass = Number of moles × Molecular mass
Mass = 0.10 × 17
Mass = 1.7 grams
Therefore, 1.7 grams are there in 0.10 moles of OH ions.
To learn more about number of moles refer- https://brainly.com/question/15356425
#SPJ1
Which of the following units is used when measuring electrical power?
Answers
Answer:
Joules/sec and watts.
Explanation:
1 Joule/sec is a rate of energy expending every second. It is equal to a watt, the English term.
What do understanding isotopes explain on the periodic table 
Answers
when a weak acid react with a weak base. what's the result
Answers
Answer:
weak acids and weak bases only ionize partially. Therefore, when they are mixed, it generally results in a reversible reaction with the formation of a conjugate acid and a conjugate base as products.Weak acid-weak base neutralization reaction in which a weak acid reacts with a weak base to form a neutral salt and water.What is the most stable form of Calcium in terms of nuclear stability?
Answers
Answer
Calcium - 40 is the most stable and most likely to be found occurring naturally
Explanation
Nuclear stability means that the nucleus of the element is stable enough to not decay with the passing of time, this decay is known as radioactivity. When it comes to Calcium, we have many isotopes of this element, but only a few have such a long half-life that we can consider them to be stable, and Calcium - 40 is the most stable and common of them.