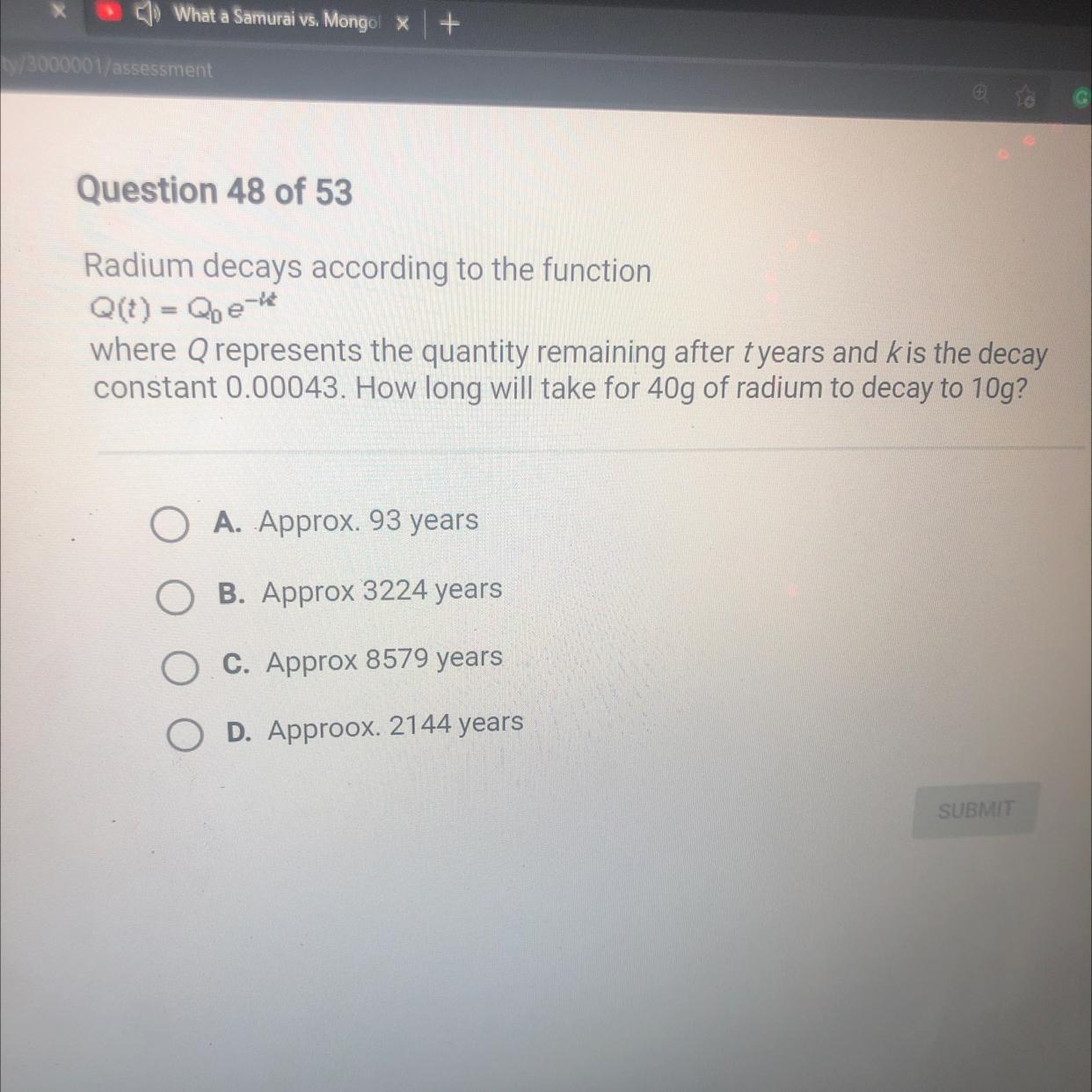
Answers
First, we have the next data:
Q(t) = final mass at time t = 10 g
Qo = initial mass = 40 g
v = decay constan = 0.00043 1/years
This kind of process like decay, follow a first-order reaction, and the formula used for this:
[tex]\begin{gathered} Q(t)=Qoxe^{-vxt} \\ \text{Clearing t:} \\ \frac{Q(t)}{Qo}=e^{-vxt} \\ \ln (\frac{Q(t)}{Qo})=-\text{vxt} \\ \frac{\ln (\frac{Q(t)}{Qo})}{-\text{v}}=t \end{gathered}[/tex][tex]\begin{gathered} \frac{\ln (\frac{10}{40})}{-0.00043\text{ 1/years}}=\text{ t} \\ \end{gathered}[/tex]Answer: t = 3224 years
Related Questions
Using the Gay-Lussac LawIf I have 7.7 moles of gas at a pressure of 0.09 atm at a temperature of 56 C°, what is the volume of the container that the gas is in?
Answers
ANSWER
EXPLANATION
Given that;
The number of moles of the gas is 7.7 moles
The pressure of the gas is 0.09 atm
The temperature of the gas is 56 degrees Celcius
Follow the steps below to find the volume of the gas in the container
Step 1; Convert the temperature to degrees Kelvin
[tex]\text{ T K = t}\degree C\text{ + 273.15}[/tex][tex]\begin{gathered} \text{ T K = 56 + 273.15} \\ \text{ T K = 329.15K} \end{gathered}[/tex]Step 2; Apply the ideal gas equation
[tex]\text{ PV = nRT}[/tex][tex]\begin{gathered} \text{ Recall, that R = 0.08205 L atm mol}^{-1}K^{-1} \\ \text{ 0.09 }\times\text{ V = 7.7 }\times\text{ 0.08205 }\times\text{ 329.15} \\ \text{ 0.09V = 207.95} \\ \text{ Divide both sides by 0.09} \\ \text{ V = }\frac{\text{ 207.95}}{\text{ 0.09}} \\ \text{ V = 2310.58 Liters} \end{gathered}[/tex]Therefore, the volume of the container is 2310.58 liters
The half-life for the first order radioactive decay of lodine - 131 is 8.0 days. After 3 half lives, what percentage of a sample of locine-131)remains?O 50.%O 25%,O 12.5%O 1%
Answers
Answer:
12.5 %.
Explanation:
Let's see the half-life formula:
[tex]N(t)=N_0\cdot(\frac{1}{2})^{t\text{/h}}.[/tex]Where N₀ is the initial amount, t is time, and h is the half-life.
We want to know what would be the percentage of a sample of iodine-131 that remains. This is the same that N(t)/N₀, so we have to replace the given data in the formula and multiply it by 100 because it is a percentage.
The half-life of iodine-131 is 8.0 days, and 3 half-lives are equal to 24 days (8.0 x 3 = 24):
[tex]\begin{gathered} \frac{N(t)}{N_0}=(\frac{1}{2})^{t\text{/h}}, \\ (\frac{1}{2})^{24\text{/8}}=(\frac{1}{2})^3=\frac{1}{8}=0.125. \\ We\text{ want the result in percentage, so multiplying by 100}\%,\text{ we obtain:} \\ 0.125\cdot100\%=12.5\%. \end{gathered}[/tex]The answer would be 12.5 %.
Adding up all of the _______ of all of the individual atoms within one molecule of a compound will determine the molecular mass of the compound.A) atomic massesB) electronsC) atomic numbersD) protons
Answers
1) Subatomic particles. There are three main subatomic particles. These subatomic particles are the protons, the neutrons, and the electrons.
The protons and the neutrons are a lot heavier than the electrons. So, they represent most of the weight of an atom.
The protons and the neutrons added together are what we call the mass number.
The correct answer is option A, atomic masses.
HURRY! NEED WITHIN 10 MINS
Answers
The equation of photosynthesis is shown in the image attached to this question.
What is photosynthesis?The term photosynthesis has to do with a kind of reaction that helps plants to make their own food. The process involves the combination of carbon dioxide and water in the presence of sunlight to give glucose and water only.
We know that the green plants are autotrophic thus they are able to make their own food. The process of photosynthesis produces the food that is passed along the food chain in the ecosystem.
Learn more about photosynthesis:https://brainly.com/question/1388366
#SPJ1
A neutral atom of on element has two electrons
with n=1, eight electrons with n=2, eight electrons
with n=3 and one electron with n =4 and has mass
number of 39. Deduce the following from the above
information
i the atomic number of the element.
is number of neutrons in the nucleus
i total number of s electrons.
iv total number of p electrons .
v the group the element belongs to
Answers
A neutral atom of on element has two electrons with n=1, eight electrons with n=2, eight electrons with n=3 and one electron with n =4 and has mass number of 39 then the atomic number of element is 39 and number of neutron in the nucleus is 50 and total number of s electron is 2 and total number of p electron is 8 and the group is 3rd group
Yttrium is a metallic element with atomic number 39 usually included in the rare earth group that occur usually with other rare earth element in minerals and is used especially in phosphorous and YAG laser and alloy etc and element with mass number of 39 is yttrium and the atomic number of element is 39 and number of neutron in the nucleus is 50 and total number of s electron is 2 and total number of p electron is 8 and the group is 3rd group
Know more about element
https://brainly.com/question/12054275
#SPJ1
A piece of sodium metal reacts completely with water. The hydrogen gas generated is collected over water at 25oC. The volume of the gas is 252mL measured at 1.00atm. Calculate the number of grams of hydrogen that was collected
Answers
The mass or number of grams of hydrogen that was collected in the experiment is 0.02 g.
What is the number of moles of hydrogen gas collected at the given conditions of temperature and pressure?The number of moles of hydrogen gas collected at the given conditions of temperature and pressure is calculated using the ideal gas equation as follows:
PV = nRT
Where;
P is pressure = 1.00 atm
V is volume = 252 mL or 0.252 L
n is the number of moles = ?
R is molar gas constant = 0.082 L.atm/K.mol
T is temperature = 273 + 25 K
T = 298 K
n = PV/RT
n = 1 * 0.252 / (298 * 0.082)
n = 0.01 moles
1 mole of hydrogen gas has a mass of 2.0 g
mass of 0.01 moles of hydrogen = 2 * 0.01
Mass of 0.01 moles of hydrogen = 0.02 g
Learn more about ideal gas equation at: https://brainly.com/question/27870704
#SPJ1
Predict the formula for each ionic compoundbarium sulfate:lithium nitrate :cesium sulfide:
Answers
INFORMATION:
We have the following ionic compounds:
- barium sulfate
- lithium nitrate
- cesium sulfide
And we must write their formulas
STEP BY STEP EXPLANATION:
1. Barium sulfate:
To write its formula, we need to use that:
- the element symbol for Barium is Ba, and it has a charge of 2+
- in the given table, sulfate is a polyatomic ion represented by SO4, and it has a charge of 2-
Now, we must add up the charges to verify if the ionic compound is neutral
[tex]2^++2^-=0[/tex]Since the compound has a net charge of zero, we can write its formula as
[tex]BaSO_4[/tex]2. Lithium nitrate:
To write its formula, we need to use that:
- the element symbol for Lithium is Li, and it has a charge of 1+
- in the given table, nitrate is a polyatomic ion represented by NO3, and it has a charge of 1-
Now, we must add up the charges to verify if the ionic compound is neutral
[tex]1^++1^-=0[/tex]Since the compound has a net charge of zero, we can write its formula as
[tex]LiNO_3[/tex]3. Cesium sulfide:
To write its formula, we need to use that:
- the element symbol for Cesium is Cs, and it has a charge of 1+
- the element symbol for sulfide is S, and it has a charge of 2-
Now, we must add up the charges to verify if the ionic compound is neutral
[tex]1^++2^-=1^-[/tex]Since the compound has a net different from zero, we need to analyze the compound.
So, if we have just one atom of Cs, the charge would be 1+, but if we take two atoms the charge would be 2(1+) = 2+
Now, verifying the net charge
[tex]2^++2^-=0[/tex]Now, we can write its formula as
[tex]Cs_2S[/tex]ANSWER:
barium sulfate:
[tex]BaSO_4[/tex]lithium nitrate :
[tex]LiNO_3[/tex]cesium sulfide:
[tex]Cs_2S[/tex]An aqueous solution of a nonionic (that is, nonelectrolyte) solute has a freezing point of -0.435 °C. The solution contains 2.49 g solute dissolved in 95.0 g H2O. What is the molar mass of the solute? g/mol
Answers
To answer this question, we have to use the formula of the cryoscopic descent:
[tex]\Delta T_c=k_f\cdot m[/tex]Where ΔTc is the difference between the freezing points of the pure solvent and the solution, kf is the cryoscopic constant (that for water it has a value of 1.86°C*kg/mol and m is the molality of the solution.
The first step we have to follow is to find the molality of the solution using this formula and the given values.
Estimate the difference between the freezing point of the water and the one of the solution:
[tex]\Delta T_c=0\degree C-(-0.435\degree C)=0.435\degree C[/tex][tex]\begin{gathered} 0.435\degree C=1.86\degree Ckg/mol\cdot m \\ m=\frac{0.435\degree C}{1.86\degree Ckg/mol} \\ m=0.234mol/kg \end{gathered}[/tex]The molality of the solution is 0.234mol/kg.
Now we have to use this molality to find the amount of moles present in the solution.
Molality equals moles of solute/kg of solvent. We know that there are 95.0g of solvent, by converting this mass to kg and using the molality we can find the amount of moles of solute present in the solution, this way:
[tex]95.0g\cdot\frac{1kg}{1000g}=0.095kg[/tex][tex]0.095kg\cdot\frac{0.234mol}{kg}=0.02223mol[/tex]It means that there are 0.02223 moles of solute in the solution.
Our last step to answer this question is to divide the mass of solute present in the solution by the amount of moles of it to find its molar mass:
[tex]\frac{2.49g}{0.02223mol}=112.01g/mol[/tex]It means that the molar mass of the solute is 112.01g/mol.
What is the pH of a solution where [OH-] = 5.6 × 10-4 M?
Answers
The pH value indicates the concentration of H+ ions in the solution. We are given the value of the concentrations of the OH- ions, so we can find the pOH using the following equation:
[tex]pOH=-log\lbrack OH^-\rbrack[/tex]The sum of pH and pOH will always be equal to 14, so we find the pH by clearing it from the following relationship:
[tex]pH+pOH=14[/tex]So, pOH value will be:
[tex]pOH=-log\lbrack5.6\times10^{-4}\rbrack=3.3[/tex]The pH will be:
[tex]\begin{gathered} pH=14-pOH \\ pH=14-3.3=10.7 \end{gathered}[/tex]The pH of the solution will be 10.7
4.A gas occupies 8.7L at a temperature of 29.0°c. What is thevolume at 133°C? (Charles Law)
Answers
11.7L
Explanations
According to Charles law, the volume of a given mass of gas is directly proportional to its temperature provided that the pressure is constant. Mathematically;
[tex]\begin{gathered} v\alpha T \\ v=kT \\ k=\frac{v_1}{T_1}=\frac{v_2}{T_2} \end{gathered}[/tex]where:
• v1 and v2 are the ,initial and final ,volume
,• T1 and T2 are the, initial and final, temperature
Given the following parameters
v1 = 8.7L
T1 = 29.0°C = 29 + 273
T1 = 302K
T2 = 133+ 273 = 406K
Substitute the given parameters into the formula
[tex]\begin{gathered} v_2=\frac{v_1T_2}{T_1} \\ v_2=\frac{8.7L\times406}{302} \\ v_2=\frac{3532.2}{302} \\ v_2=11.7L \end{gathered}[/tex]Hence the volume of the gas at 133°C is 11.7L
What part of a cell provides instructions for the processes within the cell
DNA _ Chloroplasts _ Cell wall or Glucose
Answers
The information for the cellular process is provided by DNA. DNA has genes on it, so by expressing the gene, the characteristics are expressed in the cell.
What is a gene?
Genes are present in the DNA of both prokaryotes and eukaryotes. The information of the cell is present in the gene, and it is expressed by the processes of transcription and translation in the cell.
Because of differences in gene expression, different cells, such as the nerve cell, the cardiac cell, the epithelial cell contain different proteins. Not all genes are expressed in all cells of the body. Only those genes are expressed that are transcriptionally activated and make mRNA.
Hence, DNA provides the information to the cell for the cellular process.
Learn more about the gene, here
https://brainly.com/question/411085
#SPJ1
Answer the following question:Reminder R= .0821. If you have a gas at 6 Atm and 4 L, how many moles are present if the temp is 300 K?
Answers
Answer
0.975609756 mol
Explanation
Given:
Pressure, P = 6 atm
Volume, V = 4 L
Temperature, T = 300 K
Molar gas constant, R = 0.082
What to find:
The moles, n present.
Step-by-step solution:
The mole, n present can be calculated using the ideal gas equation, PV = nRT
6 x 4 = n x 0.082 x 300
24 = 24.6n
Diivide both sides by 24.6
24/24.6 = 24.6n/24.6
n = 0.975609756 mol
Hence, the moles that are present = 0.975609756 mol
6. A sample of nitrogen gas weighs 130 g. Write the chemical formula for nitrogen gas. Howmany molecules of elemental nitrogen is this? How many atoms of nitrogen are in this sample?
Answers
Chemical formula for nitrogen gas is N₂.
To find the number of molecules in the given sample, we have to convert the mass of the sample to moles by using the molecular mass of elemental nitrogen (N₂).
[tex]130gN_2\cdot\frac{1molN_2}{28gN_2}=4.64molN_2[/tex]Now, we have to use Avogadro's number (6.022x10^23) that indicates the number of molecules in one mole of substance:
[tex]4.64molN_2\cdot\frac{6.022\times10^{23}molecules}{1molN_2}=2.79\times10^{24}molecules[/tex]It means that there are 2.79x10^24 molecules of elemental nitrogen.
To find the number of atoms we just have to multiply the number of molecules by 2, which is the number of atoms of nitrogen per molecule of elemental nitrogen:
[tex]2.79\times10^{24}molecules\cdot\frac{2atoms}{1molecule}=5.59\times10^{24}atoms[/tex]There are 5.59x10^24 atoms of nitrogen in the sample.
The energy of a photon that has a wavelength of 8.33 × 10^-6 m is ________ J.A) 2.20 × 10^-26 B) 3.60 × 10^13C) 2.39 × 10^-20 D) 2.7 × 10^9E) 4.5 × 10^-25
Answers
The energy of a photon can be found using the following equation:
What we're going to do is to replace the values of the constant and the value of the wavelength in the expression above. This is:
Therefore, the correct answer option is C. 2.39 × 10^-20 J.
Explain the role electronegativity plays in the polarity of a molecule.
Answers
Electronegativity is the force of an atom to attract electrons to itself, and Polarity represents the distribution of electron density of a molecule.
When the atoms of a molecule have very different electronegativities, zones with different electron densities are generated, and poles are formed.
So, the role that electronegativity plays in the polariry of a molecule is very important because knowing the electronegativity of atoms we can know if a molecule is polar or nonpolar.
Based on Table H, what is the vapor pressure of CH3COOH at 90.°C?
A 150 kPa
B 114 kPa
40 kPa
48 k
Answers
The equilibrium vapor pressure provides information about the rate of evaporation of a liquid. The vapor pressure of CH₃COOH at 90.°C is 40 kpa.
What is vapor pressure ?
Pressure of vapor or equilibrium the pressure that a vapor exerts on its condensed phases in a closed system when they are in thermodynamic equilibrium with one another at a specific temperature is known as vapor pressure.
It is significant to remember that when a liquid boils, the pressure of its vapor equals the atmospheric pressure. For instance, water's vapor pressure is 1 atmosphere when it boils at sea level since the surrounding pressure is also 1 atmosphere.
Thus, the vapor pressure of CH₃COOH at 90.°C is 40 kpa.
To learn more about vapor pressure follow the link below;
https://brainly.com/question/11864750
#SPJ1
Why are triple bonds shorter than single bonds?
Answers
Due to the inverse relationship between bond length and bond strength, triple bonds, which are the strongest bonds, also have the shortest bond lengths.
Why do single bonds tend to be longer than triple bonds?
The strength of the bond affects how long the bond lasts. The link will be stronger and last longer. Because they are the strongest and shortest, triple bonds. Next are double bonds, which are between single and triple bonds in terms of strength.
What produces bonds with reduced lengths?The quantity of bound electrons determines how long the bond will be (the bond order). The attraction between the two atoms is stronger and the bond length is shorter the higher the bond order.
To know more about bonds visit:-
https://brainly.com/question/10777799
#SPJ1
I really need help on this problem for chemistry, please help!
Answers
answer and explanation
we are given the balanced reaction and so we begin by calculating the number of mols of each reactant
for Al
mols = mass/Molar mass
= 22.0g / 26.98g/mol
= 0.82 mols
for oxygen
mols = mass / Molar mass
= 26.0g / 16.00g/mol
= 1.6 mols
theerefore
a. the limiting reagent is Al
b. theoretical yield will be determine using the limiting reagent.
from the balanced reaction we see that the mol ratio between aluminum and aluminum oxide is 4:2
therefore
2/4 x0.82 = 0.41 mols of aluminum oxide will form
the mass will be
mass = n x M
= 0.41 mols x 101.96g/mol
= 41.8 grams
c. percentage yield is:
actual yield/ theorectical yield x 100
= 40.0/41.8 x 100%
= 95.7 %
how many litres of gas is in 8.3 mol N2O in STP
Answers
According to STP, and furthermore, we assume ideal gas conditions:
1 mol N2O = 22.4 L
Procedure:
1 mol N2O -------------- 22.4 L
8.3 moles N2O------------- X
X= 186 L (approx.)
Answer: 186 L
Question 2 of 10Which of the following substances is a chemical that is made only bychemists?A. BleachB. CopperOC. WaterO D. Nitrogen
Answers
Answer: The best option to answer the question is letter A (bleach)
Explanation:
The question requires us to choose, among the options given, which one corresponds to a chemical compound that is only made by chemists.
Nitrogen (N2) is a gas found in our atmosphere, corresponding to 78% (by volume) in dry air. Therefore, N2 is found in nature.
Water (H2O) is also found in nature, as it is present in our atmosphere, rivers, lakes and oceans (among others).
Copper (Cu) is a metal that is also found in nature, occuring naturally in metal form and being present in minerals.
On the other hand, bleach corresponds to a mixture of chemicals where the main ingredient is sodium hypochlorite.
Therefore, among the options given, bleach is the only one that is made only by chemists (and it is not found as it is in nature).
The best option to answer the question is letter A (bleach).
The mass number is used to calculate the numner of ____ in one atom of an element. In order to calculate the number of neutrons you must subtract the ___ from the ___
Answers
2) atomic number
3) atomic mass
Determine the molar mass of an unknown monoprotic acid to two decimal places if 16.98 mL of a 0.086 M NaOH solution were used to titrate 0.236 g of the unknown acid
Answers
1 ) Chemical equation
[tex]\text{NaOH + HX}\rightarrow H_2O+Na^++X^-[/tex]HX represents the unknown acid.
2) Moles of NaOH in the reaction
[tex]M=\frac{\text{moles of solute}}{\text{liters of solution}}[/tex]Convert mL into L
[tex]L=16.98mL\cdot\frac{1L}{1000mL}=0.01698L[/tex]Plug in known values in the equation and solve for moles.
[tex]0.086M=\frac{\text{moles of NaOH}}{0.01698L}[/tex][tex]\text{mol NaOH= 0.086M}\cdot0.01698L=0.00146028\text{ mol NaOH}[/tex]3) Moles of the unknown acid that reacted with 0.00146028 mol NaOH
Molar ratio
1 mol NaOH: 1 mol HX
[tex]\text{mol HX=0.00146028 mol NaOH}\cdot\frac{1\text{ mol HX}}{1\text{ mol NaOH}}=0.00146028\text{ mol HX}[/tex]4) Molar mass of the unknown monoprotic acid
[tex]\text{Molar Mass=}\frac{\text{mass of solute (g)}}{moles\text{ of solute}}[/tex]Plug in known values and solve
[tex]\text{Molar Mass}=\frac{0.236\text{ g HX}}{0.00146028\text{ mol HX}}=161.61\text{ g/mol}[/tex]The molar mass of the unknown monoprotic acid is 161.61 g/mol
.
How many moles are in 91.5 grams of Helium?
Answers
In order to find the number of moles with a given mass of Helium, we need to use its molar mass, which is 4.0026g/mol, therefore we will have:
4.0026g = 1 mol of Helium
91.5g = x moles of Helium
x = 22.86 moles of Helium in 91.5 grams
A sample of an unknown gas with a molar mass of 85.74 is placed in a vessel with avolume of 1,681 mL at a temperature of 58.6 °C. If the pressure is 5.4 atm, howmany grams of this gas are present?
Answers
Answer
28.58 grams
Explanation
Given:
Molar mass of the gas, M = 85.74 g/mol
Volume, V = 1,681 mL = 1.681 L
Temperature, T = 58.6 °C = (58.6 + 273.15 K) = 331.75 K
Pressure, P = 5.4 atm
What to find:
The mass of the gas in grams present.
Step-by-step solution:
The mass in grams of the gas present can be calculated using the ideal gas equation:
[tex]\begin{gathered} PV=nRT \\ \\ n=moles=\frac{Mass}{Molar\text{ }mass} \\ \\ \Rightarrow PV=\frac{Mass}{Molar\text{ }mass}RT \end{gathered}[/tex]Putting the values of the given parameters and R = 0.0821 atm•L/mol•K into the formula:
[tex]\begin{gathered} 5.4atm\times1.681L=\frac{Mass}{85.74g\text{/}mol}\times0.0821atm•L/mol•K\times331.75K \\ \\ 9.0774atm•L=Mass(0.317665908atm•L/g) \\ \\ Divide\text{ }both\text{ }sides\text{ }by\text{ }0.317665908atm•L/g \\ \\ \frac{9.0774atm•L}{0.317665908atm•L/g}=\frac{Mass(0.317665908atm•L)}{0.317665908atm•L\text{/}g} \\ \\ \Rightarrow Mass=28.58\text{ }grams \end{gathered}[/tex]The mass of the gas in grams present = 28.58 grams.
Which compound BH3 or BO3 would have polar covalent bonds? How do you know?These are the options in photo
Answers
To solve this problem, let's find the electronegativity difference that we have between each pair of elements. If we look for the electronegativity of B, H and O we might find these values:
B: 1.5 O: 3.4 H: 2.1
So the two bonds that are present in our molecules have a difference of:
O ---- B = 3.4 - 1.5 = 1.9
H ----- B = 2.1 - 1.5 = 0.6
We have two types of covalent bonds, polar and non-polar. When the electronegativity difference is between 1.5 and 0.4 the bond is covalent polar, when it is less that 0.4 is non-polar and when it is higher than 1.5 it is ionic.
In our case, the electronegativity difference between O and B is 1.9, it seems that it would be an ionic bond. But that's not an option. The difference between H and B is 0.6, the polarity of that bond is weak compared to the polarity of the bond between O and B (if we consider that it is covalent).
So the answer to our problem is the first one:
BO₃ would have polar covalent bonds because
When reacting 5.00 g of MgCl2 with 15.0 g of AgNO3 , what is the limiting reagent based on the following equation?MgCl2 (aq) + 2 AgNO3(aq) --> 2 AgCl (s) + Mg(NO3)2 (aq)
Answers
Answer:
[tex]AgNO_3\text{ is the limiting reagent}[/tex]Explanation:
Here, we want to get the limiting reactant
The limiting reactant is the reactant that would produce less amount of the solid precipitate
Firstly, we need to get the number of moles of each of the reactants
To get this, we divide their masses by the molar masses
The molar mass of magnesium chloride is 95 g/mol
The number of moles would be:
[tex]\frac{5}{95}\text{ = 0.053 mol}[/tex]Now, from the equation of reaction, 1 mole of MgCl2 produced 2 moles of AgCl
Then: 0.053 mole of MgCl2 will produce 2 * 0.053 mol = 0.106 mol AgCl
For AgNO3, the molar mass is 170 g/mol
The number of moles would be:
[tex]\frac{15}{170}\text{ = 0.088 mol}[/tex]Now, looking at the equation of reaction:
2 moles of AgNO3 produce 2 moles of AgCl
0.088 mol AgNO3 will also produce 0.088 mol AgNO3
Now, looking at the values of the number of moles of AgCl produced, we can see that AgNO3 produces less of the product
This means that AgNO3 is the limiting reactant
5. You have 7 moles of NaCl. How many particles are present?
Answers
1) List the known and unknown quantities.
Sample: 7 mol NaCl
Particles: unknown
2) Convert moles of NaCl to particles of NaCl.
Avogadro's number is 6.022*10^23
1 mol = 6.022*10^23
[tex]particles\text{ }NaCl=7\text{ }mol\text{ }NaCl*\frac{6.022*10^{23}\text{ }particles}{1\text{ }mol\text{ }NaCl}=4.21*10^{24}\text{ }particles[/tex]7 mol NaCl is equal to 4.21*10^24 parcicles.
How many total atoms are there in 19.3 g of hydrazine (N_{2}*H_{2})
Answers
Given the following parameters
Mass of hydrazine = 19.3 grams
Determine the moles of hydrazine
[tex]\begin{gathered} moles\text{ of N}_2H_2=\frac{mass}{molar\text{ mass}} \\ moles\text{ of N}_2H_2=\frac{19.3}{30.02928} \\ moles\text{ of N}_2H_2=0.6423moles \end{gathered}[/tex]According to the Avogadro's constant
[tex]1mole\text{ of a substance}=6.02\times10^{23}atoms[/tex][tex]\begin{gathered} atoms\text{ of N}_2H_2=0.643\times6.02\times10^{23} \\ atoms\text{ of N}_2H_2=3.87\times10^{23}atoms \end{gathered}[/tex]Therefore the total atoms that are there in 19.3 g of hydrazine is 3.87 * 10^23 atoms
2. The following mistakes were made when carrying out the experiment. What effect does each have on the
calculated molar mass? Be specific. For example, too large because...
a. Only part of the pipet was immersed in the boiling water, so the temperature in part of the pipet was less
than that of the water bath.
b. The mass of the condensed liquid was not determined quickly. Instead, the pipet was allowed to stand for
a while before immersing it in room temperature water and then massing the pipet.
Answers
The actual volume of pipet is 23.8901 mL
Explanation:
The formula for the density of water can be used to calculate the volume of water transferred. For which, let us first calculate the mass of water transferred by taking the difference of mass of beaker.
Initial mass of the dry beaker:
initial mass of beaker (dry) = 57.5348 g
Mass of the water:
mass of the water = final mass of the beaker - initial mass of the beaker
mass of the water = 81.3743 - 57.5348
mass of the water = 23.8395 g
The density of water at 21.5°C is 0.997882 g/mL
From the above data
The above calculations show that the volume transferred by pipet is 23.8901 mL.
For more information about volume.
https://brainly.com/question/25328286
#SPJ1
This is a 6 mark question. Please spend some time explaining it, answering it and helping me.
Answers
Answer:
Explanation:
Here, we want to explain the factors responsible for the differences in the polymers presented
We proceed as follows:
a) High melting Point
Although the two polymers contain carbon and hydrogen atoms only, the presence of hydrogen bonding in Polymer Y may lead to it having a high melting point compared to Z.
Other factors that can cause this include the presence of double bonds, aromatic groups or the availability of bulky side groups or branches in the polymer molecule Y.
These and more are responsible for the differences
b) Rigidity and Flexibility
This is due to the existence of some degree of rotation around the atoms' valency. Polymer Z would be flexible due to this.
The absence of this in Polymer Y will make this a difficulty
c) Stretching
How entangled the chain of the polymers are could be responsible for the stretching of the polymers. Since polymer Z is easily stretched, there would be a low degree of entanglement in its chain compared to polymer Y.