How many electrons are in the outermost shell of the ge4+ ion in its ground state?.
Answers
There are eighteen electrons in the outermost shell of the Ge4+ ion in its ground state.
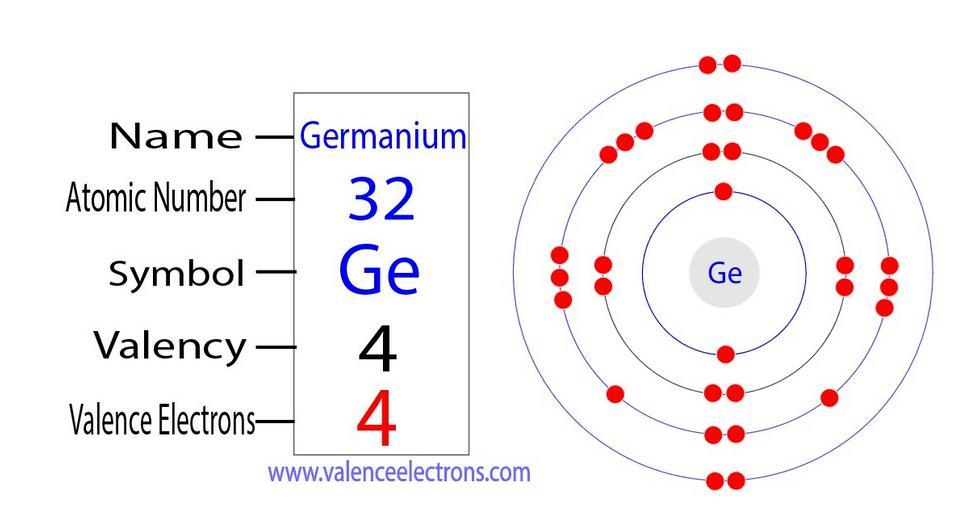
Electron configuration of germaniumThe electronic configuration shows that the last shell of germanium has four electrons and the d orbital has a total of ten electrons, but the ionic state of the element changes depending on the bond formation.
Electron configuration of the Ge4+ ionThe germanium atom donates two electrons in the 4p orbital and two electrons in the 4s orbital to convert the germanium ion (Ge4+).
Ge – 4e– → Ge4+
The electronic configuration of the germanium ion (Ge4+) is:
1s2 2s2 2p6 3s2 3p6 3d10.
Electrons in the last shell of the Ge4+ ionThe electron configuration shows that the Ge4+ ion has three shells and the last shell has 18 electrons.
In other words, there are eighteen valence electrons in the germanium Ge4+ ion.
Learn more about ions at https://brainly.com/question/14982375
#SPJ4
Related Questions
How many covalent bonds are predicted for an atom of chlorine?
Answers
Covalent bonds are predicted for an atom of chlorine = 1
Covalent bonds are made by sharing of electrons to complete the octet or doublet of the atom to attain stability.
Covalent bond refers to the mutual sharing of one or more pairs of electrons between two atoms.
These electrons simultaneously attract the atomic nuclei of the two atoms participating in the covalent bond.
A covalent bond is formed only when the electronegativity difference of two atoms is too small so that an electron transfer to form ions will not occur.
Number of bonds for a neutral atom = (Number of electrons in the complete valence shell i.e. 2 or 8 electrons) - (Number of valence electrons of the atom)
Number of bonds for chlorine = (Number of electrons in the complete valence shell i.e. 2 or 8 electrons) - (Number of valence electrons of the carbon)
which means,
Number of bonds for chlorine atom = 8 - 7
Number of bonds for chlorine atom = 1
To know more about covalent bonds:
brainly.com/question/19382448
#SPJ4
if a central atom has 6 equivalent bonds to other atoms and no extra lone pairs of electrons, then the shape of the molecule is said to be
Answers
An atom with 6 bonds without a lone pair of electrons gives an octahedral molecular shape using the VSEPR rules.
What is VSPER Theory?
Based on the number of valence shell electron bond pairs between the atoms in a molecule or ion, the valence shell electron pair repulsion (VSEPR) hypothesis is a model used to predict 3-D molecular shape. According to this hypothesis, electron pairs will position themselves to reduce the consequences of their mutual repulsion. The electron pairs are, in other words, as far apart as they can be.
For forecasting and visualising molecular structures, utilise the VSEPR model. Linear, trigonal planar, angled, tetrahedral, trigonal pyramidal, trigonal bipyramidal, disphenoidal (seesaw), t-shaped, octahedral, square pyramidal, square planar, and pentagonal bipyramidal are the different types of structures.
Learn more about VSPER Theory from given link
https://brainly.com/question/14225705
#SPJ4
we cannot specify how long an individual radioactive isotope will survive before it decays, but we can measure how long it takes for a population of the parent isotopes to be halved. this is a consequence of half of the parent isotopes decaying into daughter isotopes. this time unit is called the half-life of the isotope. for a given radioactive element, the half-life is a constant regardless of conditions. q19 the ratio of parent-to-daughter isotopes changes with the passage of each successive half-life. how many of the original atoms of a parent isotope remain unchanged after five half-lives? group of answer choices 1/4 1/8 1/16 1/32
Answers
A quantity of the original isotope (18) is kept. A particular radioactive isotope's half-life is constant; it is unaffected by environmental factors and is unrelated to the starting concentration of that isotope.
How does radioactive decay affect the parent isotopes?These isotopes emit alpha, beta, and gamma rays as a result of their radioactive decay. A decay chain starts with a parent isotope.
What exactly is an isotope's daughter?The radioactive decay of parent isotopes yields daughter isotopes. Although reactions occasionally produce stable daughter isotopes, they are typically unstable and radioactive, which promotes the progression of decay chains.
To know more about Atoms visit:-
brainly.com/question/1566330
#SPJ4
When 535) of heat is added to 8.5g of cooking oil at 22°C, the temperature increases to 79°C. What is the specific heat
of the cooking oil?
Answers
When 535) of heat is added to 8.5g of cooking oil at 22°C, the temperature increases to 79°C, 0.797 J/g°C is the specific heat of the cooking oil.
What is specific heat?The amount of heat needed to increase a substance's temperature by one degree Celsius in one gram, also known as specific heat.
According to the definition, a material's specific heat capacity is normally calculated by measuring the heat capacity of a sample of the substance, commonly using a calorimeter, and dividing by the sample's mass.
Given that,
Heat absorbed (Q) = 535 J
Mass = 8.5 gm
Change in temperature (ΔT) = 79°C
Now, Specific heat (S) is given by the formula:
S = Q/(m × ΔT)
or, S = 535 J/ (8.5 gm × 79°C)
or, S = 0.797 J/g°C.
Thus, 0.797 J/g°C is the specific heat of the cooking oil.
To know more about specific heat refer to:
https://brainly.com/question/27991746
#SPJ1
Pressure has little effect on the solubility of liquids and solids because they are almost incompressible. (True or False)
Answers
The given statement about pressure is true as per the Henry's law.
Liquids and solids showcase nearly no alternate of solubility with modifications in strain. Gases as is probably expected, growth in solubility with an growth in strain. Henry's Law states that: The solubility of a fueloline in a liquid is immediately proportional to the strain of that fueloline above the surface of the solution. External pressure has little or no impact at the solubility of drinks and solids. In contrast, the solubility of gases will increase because the partial stress of the fueloline above an answer will increase.
Therefore, the given statement is true.
To learn more about pressure check the link below:
https://brainly.com/question/28012687
#SPJ4
which of the following statements about an e1 mechanism is not true? group of answer choices the reaction is fastest with tertiary alkyl halides a better leaving group makes the reaction rate increase the reaction follows first-order kinetics stronger bases favor the e1 reaction
Answers
d) stronger bases favor the E1 reaction statement about and E1 mechanism is not true
Ionization and deprotonation are typically the two stages involved in the E1 mechanism, also known as unimolecular elimination. The production of a carbocation occurs as an intermediate during ionisation. A proton is lost by the carbocation in deprotonation. SN1 and E1 Reactions have remarkably similar processes; the outcome just depends on whether the nucleophile or the base hits first. The pace of the reaction is solely dependent on the substrate in SN1/E1 reactions as opposed to second order SN2 and E2 reactions (see "SN2 Reactions" and "E2 Reactions").
Learn more about E1 mechanism here:
https://brainly.com/question/8620318
#SPJ4
given that you wished to use exactly 0.325 mole of nel/ to prepare a 2.50 m naci solution, how many milliters of solution must you prepare?
Answers
130 ml is milliters of solution must you prepare
What is Molarity?
Molality, also known as moles per kilogramme of a solvent, is the quantity of moles of a solute that may be found in a kilogramme of the solvent.
A mole is a unit of measurement for a chemical compound, and it is from this unit that the word "molarity" is derived
It is assessed by taking into account two indications, namely the volume of the solution and the quantity of solute molecules. Essentially, the volume of the solution is determined in litres. The letter "M" stands for molarity.
130 ml Molarity = moles/liter molarity
= 2.50 moles
= .325 2.5
= .325/liters solving this equation for liters: 2.5 * liters
= .325 liters
= .325/2.5 = 0.13 now a unit conversion from liters to the milliliter unit requested by the problem.
1 liter is 1000 milliliters .130l * 1000 ml/l
= 130 ml
Learn more about Molarity from given link
https://brainly.com/question/17138838
#SPJ4
Arrange the organic compounds from most soluble in water to least soluble in water. Most soluble in water CH4 CH, OH CH,OCH, Least soluble in water
Answers
On the basis of the polarity of the given compounds the order of the solubility in water is:
CH₃OH > CH₃OCH₃ > CH₄
What is solubility?Solubility can be defined as the maximum amount of Solute that can dissolve in a known amount of solvent at a specific temperature.
The solubility of the given compound depends upon the polarity of the compound. From the concept of like dissolves like therefore the polar compound is soluble in polar solvents and nonpolar compounds in nonpolar solvents.
Water is a polar solvent and CH₃OH is also a polar compound so it can form hydrogen bonding with water. Therefore it has the highest solubility in water. Methane is nonpolar it gas lowest solubility in water.
Learn more about solubility, here:
brainly.com/question/8591226
#SPJ1
Your question was incomplete, most probably the complete question was,
Arrange the organic compounds from most soluble in water to least soluble in water. Most soluble in water CH₃OH, CH₃OCH₃, and CH₄ Least soluble in water.
a sample of phosphoric acid (h3po4) weighing 2.80 g is dissolved in 150.0 ml of 1.00 m naoh. calculate the molar concentrations of sodium ion, hydroxide ion, and phosphate ion after the reaction is complete
Answers
The molar concentrations of sodium ion, hydroxide ion, and phosphate ion after the reaction is complete is :
1) [Na] = = 1 M
2) [PO₄] = 0.191 M
3) [OH] = 0.428 M
The balanced equation is given as :
3NaOH + H₃PO₄ ----> Na₃PO₄ + 3H₂O
volume of NaOH = 150 mL = 0.150 L
molarity of NaOH = 1 M
moles of NaOH = molarity × volume
= 1 × 0.150
= 0.150 mol
moles of H₃PO₄ = mass / molar mass
= 2.80 / 97.99
= 0.0286 mol
1) [Na] = 0.150 mol / 0.150 L = 1 M
2) [PO₄] = 0.0286 mol/ 0.150 L = 0.191 M
3) [OH] = 0.150 mol - 3(0.0286 mol) = 0.0642 mol
[OH] = moles / volume
= 0.0642 / 0.150
= 0.428 M
To learn more about moles here
https://brainly.com/question/14664529
#SPJ4
a sealed cylinder fitted with a movable piston contains ideal gas at 27°c, pressure 0.500 × 105 pa, and volume 1.1 m3. what will be the final temperature if the gas is compressed to 0.800 m3 and the pressure rises to 0.820 × 105 pa?
Answers
Considering the combined law equation, the final temperature if the gas is compressed to 0.800 m³ and the pressure rises to 0.820×10⁵ Pa is 357.82 K.
Boyle's lawBoyle's law states that the pressure of a gas in a closed container is inversely proportional to the volume of the container, when the temperature is constant.
Mathematically, Boyle's law states that the product of pressure and volume always has the same value:
P×V= k
where:
P is the pressure.V is the volume.k is a constant.Charles' lawCharles' law states that the volume is directly proportional to the temperature of the gas when the pressure is constant.
Mathematically, Charles' law states that the ratio between volume and temperature always has the same value:
V÷T=k
where:
V is the volume.T is the temperature.k is a constant.Gay-Lussac's lawGay-Lussac's law states that the pressure of a gas is directly proportional to its temperature when the volume is constant.
Mathematically, Charles' law states that the ratio between pressure and temperature always has the same value:
P÷T=k
where:
P is the pressure.T is the temperature.k is a constant.Combined law equationCombined law equation is the combination of three gas laws called Boyle's, Charlie's and Gay-Lusac's law:
(P×V)÷T= k
where:
P is the pressure.V is the volume.T is the temperature.k is a constant.Analyzing an initial state 1 and a final state 2, it is fulfilled:
(P₁×V₁)÷T₁= (P₂×V₂)÷T₂
Final temperature in this caseIn this case, you know:
P₁= 0.500×10⁵ PaV₁= 1.1 m³T₁= 27 °C= 300 K (being 0 °C= 273 K)P₂= 0.820×10⁵ PaV₂= 0.800 m³T₂= ?Replacing in the combined gas law:
(0.500×10⁵ Pa× 1.1 m³)÷ 300 K= (0.820×10⁵ Pa× 0.800 m³)÷T₂
Solving:
[(0.500×10⁵ Pa× 1.1 m³)÷ 300 K]× T₂= (0.820×10⁵ Pa× 0.800 m³)
T₂= (0.820×10⁵ Pa× 0.800 m³)÷ [(0.500×10⁵ Pa× 1.1 m³)÷ 300 K]
T₂= 357.82 K
Finally, the final temperature is 357.82 K.
Learn more about combined law equation:
brainly.com/question/4147359
#SPJ1
When a highly reactive metal, such as magnesium (mg), is mixed with a reactive nonmetal such as sulfur (s), the two elements will most likely combine to form a new substance, magnesium sulfide (mgs).
Answers
when a highly reactive metal such as magnesium is mixed with a reactive nonmetal such as sulfur, the two elements will most likely combine to form a new substance, magnesium sulfide (Mgs). This type of reaction is called a chemical reaction
Chemical reaction occurs when the atoms of the reactant elements rearrange to form new substances with different chemical properties. The resulting substance, magnesium sulfide, has different chemical properties than the reactants, magnesium and sulfur. For example, magnesium sulfide is a solid at room temperature, while both magnesium and sulfur are solids at room temperature. Additionally, magnesium sulfide is not very reactive, while both magnesium and sulfur are highly reactive elements. These differences in chemical properties are a result of the chemical reaction between the two elements.
to know more about sulfur-
https://brainly.com/question/1478186
#SPJ4
draw the structure of the carbocation intermediate that leads to the indicated products in this reaction.
Answers
The two carbocation intermediates' structures could result from the reaction of 2-methyl-2-butene with HBr.
A species known as a carbocation is one in which a molecule's carbon atom has less than eight protons and a positive formal charge. It is frequently thought of as an intermediary in different reaction pathways. From tertiary to secondary to primary, carbocations lose stability.
The top structure is created when the hydrogen from HBr (explicitly depicted) is linked to the secondary carbon that was previously carrying the double bond. As a result, a tertiary carbocation will occur. The bottom structure, where the tertiary carbon contains the hydrogen and charges the secondary carbon positively, is the less stable intermediate.
Hence, tertiary carbocation is more stable than secondary and primary.
To know more about Double bond.
https://brainly.com/question/29068102
#SPJ4
combine the PV = nRT and n = m/M to solve for molar mass, M.
what is this asking and how can I do it?
Answers
Ideal gas law is valid only for ideal gas not for vanderwaal gas. Combined Boyle's and Charles' gas law is used. Therefore, the combined formula is PVM=mRT.
What is ideal gas equation?Ideal gas equation is the mathematical expression that relates pressure volume and temperature. Real gas behave like an ideal gas at low pressure and high temperature. There is no forces of attraction or repulsion between the particles of ideal gas.
The formula that comes by combining PV = nRT and n = m/M is
PV = nRT
PV =( m/M )RT
PVM=mRT
From this formula we can find the molar mass easily.
Therefore, the combined formula is PVM=mRT.
To learn more about ideal gas equation, here:
https://brainly.com/question/14826347
#SPJ1
α and β rays consist of particles, whereas γ rays are high-energy radiation and contain no particles and thus they have no mass. Given that the penetrating power decreases with mass, rank the rays according to their penetrating power. Rank the components from strongest penetrating power to weakest penetrating power.
Answers
Therefore, the following is the order of penetrating power:
The most powerful penetrating force is gamma () rays.
Between gamma and alpha rays are beta() rays.
Alpha(a) rays have the lowest penetrating power.
What is penetrating power ?
The penetration power of each form of radiation describes how well it may penetrate solid objects. The radiation's penetration power increases with the amount of material it can penetrate, making them more harmful.
Gamma rays is a photon, and it is a ray, while alpha and beta are particles. The least invasive particles are alpha rays. A sheet of paper or a few inches of air will stop them. Next are beta particles. A metal sheet can be used to halt them. The penetration power is greatest among the three for gamma rays. Lead or concrete piled several feet high will simply make them weaker.
To learn more about gamma rays click here:
https://brainly.com/question/12528914
#SPJ4
What gas occupies 22.4 at STP?
Answers
One mole of hydrogen molecules at S.T.P occupies 22.4 L volume.
To comparing various gas characteristics, standard temperature and pressure (STP) is a helpful set of reference conditions. Gases have a volume of 22.4 L per mole at STP. The ideal gas law may be used to calculate gas densities. The ideal gas law equation's variable "R" is referred to as the "gas constant." A constant number is always equal to the product of the pressure times the volume of a gas divided by the number of moles and the gas's temperature. The units that the temperature, pressure, and volume are expressed in determine the constant's numerical value.
Learn more about STP here:
brainly.com/question/15469566
#SPJ4
a current of 5.34 a is passed through a ni(no3)2 solution. how long, in hours, would this current have to be applied to plate out 8.10 g of nickel?
Answers
0.054 hr current have to be applied to plate out 8.10 g of nickel.
Electrochemistry is the branch of bodily chemistry concerned with the relationship between electric ability distinction, as a measurable and quantitative phenomenon, and identifiable chemical alternate, with the capability distinction as an final results of a specific chemical trade, or vice versa.
Electrochemistry is the observe of electron motion in an oxidation or reduction reaction at a polarized electrode floor. each analyte is oxidized or reduced at a particular capability and the current measured is proportional to attention. This method is a powerful method closer to bioanalysis.
Calculation:-
m = Zit
t = m/Zi
= 8.10/28× 5.34
= 0.054 hr
Learn more about electrochemistry here:-https://brainly.com/question/16815411
#SPJ4
balance the equation with the correct coefficients
C₂H2 +
O2 →
CO₂ +
H₂O
Answers
Answer: 2C2H2 + 5O2 → 4CO2 + 2H2O
Explanation:
How do you predict a number?
Answers
Without letting me know, choose a number and then indicate the card or cards that it appears on.
I'll tell you the number you're thinking of!
Simply look at the top left corner of the cards and add the numbers (4 + 1), for instance, if someone chooses the number 5 and points to the two cards it appears on.
Any math lesson can benefit from the energy and amazement that math magic tricks bring to the subject. You can present them to kids as problem-solving exercises and give them the task of demystifying them so they become useful exercises for fostering critical thinking abilities. THOANs are perhaps the easiest to start with (Think Of A Number).
Question:
"How can I predict the next number in a non-obvious sequence?"
To know more about chemistry, visit;
brainly.com/question/13428382
#SPJ4
a cubic unit cell contains manganese ions at the corners and fluoride ions at the center of each edge. what is the empirical formula of this compound? mn
Answers
A cubic unit cell contains manganese ions at the corners and fluoride ions at the center of each edge. Emperical formula of the coumpound is MnF₃
What is Emperical formula?
The chemical formula of a compound with an empirical formula provides the ratios of the components present in the molecule but not the precise number or arrangement of atoms. This would be the compound's element to whole number ratio with the lowest value.
The determination is empirical because it is based on experimental evidence. For instance, while glucose has the chemical formula C 6H 12O 6, the empirical formula is CH 2O.
Therefore, A cubic unit cell contains manganese ions at the corners and fluoride ions at the center of each edge. Emperical formula of the coumpound is MnF₃
To learn more about Emperical formula
Here: https://brainly.com/question/13058832
#SPJ4
Thermal energy moves from an area of. Matter to an area of matter
Answers
Answer:
Convection- the answer to the question
at a certain temperature the rate of this reaction is first order in with a rate constant of suppose a vessel contains at a concentration of . calculate how long it takes for the concentration of to decrease by . you may assume no other reaction is important. round your answer to significant digits.
Answers
1/8 of a second (2 significant figures). When this reaction occurs at a specific temperature, its rate is first order with a rate constant of let's say a concentration in a vessel.
What exactly is rate constant k?The proportionality constant (k) connecting the rate of the reaction to reactant concentrations determines the specific rate constant (SRC).
The reaction's equation is CICH2CH2Cl (g) --> CH2CHCI (g) + HCl (g)
Rate constant (k) equals 2.01 s-1
This is a first order reaction based on the rate constant's units.
Initial Concentration: 1.34 M t =
Final concentration equals 20 percent of 1.34, or 0.268 M.
First order reactions have the integrated rate rule ln[A] = ln[A]o - kt ln(0.268) = ln(1.34) - 2.01(t) -2.01(t) = - 1.6094 t = 0.8007 0.80 seconds (2 significant figures).
To know more about rate constant visit:-
https://brainly.com/question/20305871
#SPJ4
hydrogen peroxide (h2o2) is a major industrial chemical that is produced on a scale approaching 109 kg per year. it is widely used as a bleach and in chemical manufacturing processes. how do you expect its boiling point to compare with those of fluorine (f2) and hydrogen sulfide (h2s), two substances with molar masses comparable with that of hydrogen peroxide?
Answers
Hydrogen peroxide is a colorless liquid at room temperature with a bitter taste. Small amounts of gaseous hydrogen peroxide occur naturally in the air.
Since it easily breaks down into oxygen and water and releases heat, hydrogen peroxide is unstable. Despite not being flammable, it has a strong oxidising agent that, when in contact with organic material, can trigger spontaneous combustion. For medical purposes, as well as for usage as a clothing and hair bleach, hydrogen peroxide is present in many homes in low amounts (3–9%). Higher quantities of hydrogen peroxide are employed in the manufacturing of organic compounds, foam rubber, and rocket fuels in addition to being utilised as a bleach for paper and textiles.
With disinfecting, antiviral, and antibacterial properties, hydrogen peroxide is a peroxide and oxidising agent. Hydrogen peroxide's oxidising activity manifests itself upon topical administration, rinsing, and generation of free radicals, which causes oxidative damage to proteins and other biological molecules.
To know more about pathogens, click the link below;
https://brainly.com/question/13051879
#SPJ4
Is CH2O a formaldehyde?
Answers
Yes, the [tex] CH_{2}[/tex]O is the correct formula of formaldehyde.
The correct arrangement of bonds is H - C - H (=O). It is first aldehyde in the series and thus has only hydrogen atom attached to the aldehyde functional group. The valency of carbon is filled as two hydrogens form single bond and one oxygen is attached through double bonds with carbon.
Formaldehyde finds wide application in different fields as medicines, building and construction, food, manufacturing of sanitary products, disinfectants and many more. Produced by oxidation of methanol, it is a gaseous chemical with strong smell.
Learn more about formaldehyde -
https://brainly.com/question/28366450
#SPJ4
. saccharin (c7h5no3s) is sometimes dispensed in tablet form. ten tablets with a total mass of 0.5894 g were dissolved in water. this solution was then oxidized to convert all the sulfur to sulfate ion, which was precipitated by adding an excess of barium chloride solution. the mass of baso4 obtained was 0.5032 g. what is the average mass of saccharin per tablet? what is the average mass percent of saccharin in the tablets?
Answers
The moles are literally the molar mass of a solute and has the moles of solute and solvent of this solution as the average mass percent of saccharin in the tablets is 69%.
The sulfur (S) is oxidized to sulfate ions (SO₄²⁻). All the SO₄²⁻ ions are brought about with BaCl₂ answer withinside the shape of BaSO₄ (s).
The response is as follows-
BaCl₂ (aq) + SO₄²⁻ (aq) → BaSO₄ (s) + 2Cl⁻So, this shows that after 1 mol of Saccharin (C₇H₅NO₃S) is dissolved, then 1 mol of BaSO₄ is brought about.The quantity of moles of C₇H₅NO₃S is same to the quantity of moles of BaSO₄.The quantity of moles of BaSO₄ = (mass of BaSO₄ obtained) / (molecular mass of BaSO₄) = (0.5032 g) / (233.39 g/mol) = 0.0022 molesSo, The quantity of moles of C₇H₅NO₃S = 0.0022 molesMass of Saccharin (C₇H₅NO₃S) = (quantity of moles of C₇H₅NO₃S) × (molecular mass of C₇H₅NO₃S) = (0.0022 moles) × (183.18 g/mol) = 0.403 gThe common mass percent of Saccharin (C₇H₅NO₃S) in drugs may be decided by-[(mass of C₇H₅NO₃S) / (mass of tablets)] × 100 % = [ (0.403 g) / (0.5894 g) ] × 100 % = 68.37 %68.37n be rounded off as 69 %So, the common mass percent of Saccharin (C₇H₅NO₃S) in drugs is 69 %.Read more about mass;
https://brainly.com/question/15356425
#SPJ4
If the bond angle between two adjacent hybrid orbitals is 120°, which is the hybridization?
A. sp3d2
B. sp
C. sp3
D. sp2
Answers
The hybridization, or sp2 hybridization, is indicated if the connection angle between the two neighboring hybrid orbitals is 120 degrees.
What is hybridization, in your opinion?The process of hybridization is the mixing of orbitals that share the same energy state to create an equal number of new hybrid orbitals. This mixing usually produces hybrid orbitals with completely distinct energies, geometries, etc.
What does SP3, SP2, and sp3 hybridization entail?Sp hybridization happens whenever an orbital with an's' and a 'p' join. Additionally, the result of the combination of one's' and three 'p' orbitals is sp2 hybridization. Finally, sp3 hybridization will occur when one's' and three 'p' orbitals join.
TO know more about hybridization visit:
https://brainly.com/question/13978941
#SPJ4
a certain mineral crystallizes in the cubic unit cell shown below. m represents the cations and a represents the anions. what is the empirical formula of the mineral?
Answers
The empirical formula of the mineral is MA
The simplest whole number ratio of atoms in a compound serves as the empirical formula for that compound. Sulfur monoxide and disulfur dioxide both have empirical formulas that are as straightforward as SO and S2O2, respectively. This provides a straightforward illustration of the idea. Thus, the empirical formula for the sulphur and oxygen compounds sulphur monoxide and disulfur dioxide is the same. The number of atoms in each molecule of a chemical compound is expressed by its molecular formula, which is different for the two substances. The arrangement. or number. of atoms. are not mentioned. in an empirical. formula. It is typical for numerous macromolecules, including silicon dioxide, as well as ionic chemicals like calcium chloride (CaCl2) (SiO2).
Learn more about empirical formula here:
https://brainly.com/question/14044066
#SPJ4
calcium metal crystallizes in fcc lattice unit cell. the density of the solid is 1.54 g/cm^3. what is the radius of a calcium atom
Answers
The radius of a calcium atom is 1.97 x 10^-8 cm.
What is lattice energy?
Lattice energy is the amount of energy needed to change one mole of an ionic solid into its gaseous ionic components. It can also be described as the amount of energy required to endothermically split one mole of an ionic crystal into gaseous ions in a vacuum.
What is radius?
The atomic radius is determined by measuring the distance between an atom's nucleus and its outermost shell.
Mass of 4 Ca atoms in fcc unit cell = 4 x 40.08/6.023 x 10^23
= 2.66 x 10^-22 g/unit cell
Volume of unit cell = 2.66 x 10^-22/1.54
= 1.73 x 10^-22 cm^3
Edge length (d) = cube rt.(1.73 x 10^-22)
= 5.57 x 10^-8 cm
radius of calcium atom = 5.57 x 10^-8/(sq.rt.(8))
= 1.97 x 10^-8 cm
Therefore, the radius of a calcium atom is 1.97 x 10^-8 cm.
Learn more about lattice energy from the given link.
https://brainly.com/question/16552951
#SPJ4
What patterns do you notice about the number of valence electrons?
Answers
You can notice about the number of valence electrons that As you move across each column, the valence electron number rises by one.
Position on the periodic table of the elements is a sign of how many valence electrons are present in an atom. In groups 1-2 and 13–18 of the periodic table, the number of valence electrons increases by one from one element to the next across each row, or period.
All the elements have the same number of valence electrons inside each column, or group, of the table. This explains why all the elements in a group represent a lot of the same chemical characteristics.
So, In relation to the number of valence electrons, you can see that it increases by one as you move across each column.
To learn more about Valence electrons, Here :
https://brainly.com/question/19595162?referrer=searchResults
#SPJ4
a nuclear fission chain reaction where uranium-235 is bombarded with neutrons produces barium142, krypton-91 and which other type of product?
Answers
A nuclear fission chain reaction of uranium-235 is bombarded with neutrons and produces barium142, krypton-91, and three neutrons.
What is a nuclear chain reaction?In nuclear physics, a nuclear chain reaction takes place when one single nuclear reaction causes an average of subsequent nuclear reactions, thus leading to the possibility of a self-propagating series of these reactions. The specific nuclear reaction can be defined as the fission of heavy isotopes (e.g., uranium-235, ²³⁵U).
Fission chain reactions take place due to interactions between neutrons and fissile isotopes (such as ²³⁵U). The chain reaction needs both the emit of neutrons from fissile isotopes undergoing nuclear fission and the absorption of some of these neutrons in fissile isotopes.
[tex]^{235}U +\;^1n_0 \longrightarrow \; ^{141}Ba_{56} +^{92}Kr_{36} +3^1n_0 +\;Energy[/tex]
Therefore, three neutrons produce along with Barium 142, Krypton-91.
Learn more about nuclear chain reaction, here:
https://brainly.com/question/24297620
#SPJ1
determine the mass of fe2(so4)3 that is required to produce 1.15 mol fe3 . a. 0.575 g fe2(so4)3 b. 119 g fe2(so4)3 c. 208 g fe2(so4)3 d. 230 g fe2(so4)3 e. 460 g fe2(so4)3
Answers
The mass of fe2(so4)3 that is required to produce 1.15 mol fe3 is 230 gm.
The molar mass of a chemical compound is described as the mass of a sample of that compound divided by using the amount of substance that is the wide variety of moles in that pattern, measured in moles. The molar mass is a bulk, no longer molecular, an asset of a substance.
The molar mass of a substance is the mass in grams of one mole of the substance. As shown in this video, we are able to attain a substance's molar mass by summing the molar hundreds of its thing atoms. we will then use the calculated molar mass to convert among mass and variety of moles of the substance.
Calculation:-
Fe₂(SO₄)₃ -------> 2Fe₂⁺³ + 3SO₄⁻²
2 mole Fe⁺³ produced by 1 mole fe2(so4)3
1.15 mol Fe⁺³ produced by = 1/2 × 1.15
= 0.575 mol
molar mass of fe2(so4)3 = 400 g
mass of 0.75 moles of fe2(so4)3 is = 400 × 0.575
= 230 gm
Learn more about molar mass here:-https://brainly.com/question/837939
#SPJ4