Answers
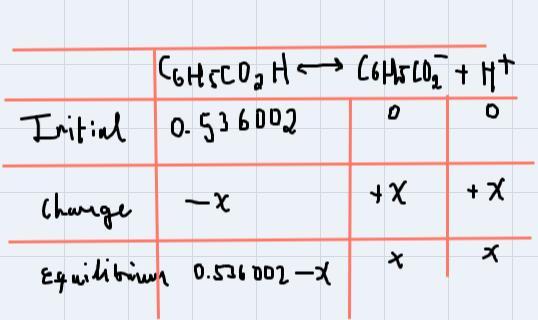
To solve this problem, we could use the ICE table as follows:
First of all, the reaction that we're working with is:
[tex]C_6H_5CO_2H\leftrightarrow C_6H_5CO^-_2+H^+_{}[/tex]Now, let's make the table:
As you can notice, the initial concentration of C6H5CO2H is 0.536002M, and as the products haven't been formed yet, their concentration is 0.
When the change happen, the concentration of the reactant will decay "x" and the concentration of the products will increase "x". (That's the reason of the signs).
And finally, the equilibrium.
We also know that:
[tex]K_a=6.5\cdot10^{-5}[/tex]And, this equilibrium constant comes from the following:
[tex]K_a=6.5\cdot10^{-5}=\frac{\lbrack H^+\rbrack\lbrack C_6H_5CO^-_{2^{}}\rbrack}{\lbrack C_6H_5CO_2H\rbrack}[/tex]Where all concentrations are the equilibrium concentrations.
If we replace, we got that:
[tex]\begin{gathered} 6.5\cdot10^{-5}=\frac{\lbrack H^+\rbrack\lbrack C_6H_5CO^-_{2^{}}\rbrack}{\lbrack C_6H_5CO_2H\rbrack} \\ \\ 6.5\cdot10^{-5}=\frac{x\cdot x}{0.536002-x} \\ \\ 6.5\cdot10^{-5}=\frac{x^2}{0.536002-x} \end{gathered}[/tex]We're going to solve this equation for x:
[tex]\begin{gathered} 6.5\cdot10^{-5}(0.536002-x)=x^2 \\ =3.484013\cdot10^{-5}-6.5\cdot10^5x-x^2=0 \end{gathered}[/tex]And, using the quadratic formula we obtain that the value of x is 0.000587014 approximately.
Now, if x=0.000587014, that means that [H+] = 0.000587014
And finally, remember that the pH of a solution is defined as:
[tex]\begin{gathered} pH=-\log \lbrack H^+\text{\rbrack} \\ pH=-\log \lbrack0.000587014\rbrack \\ pH=3.23 \end{gathered}[/tex]Therefore, the pH is 3.23.
Related Questions
What would the molecular formula be if potassium and chlorine reacted to form a neutral compound?A. KClB. PClC. K2ClD. KCl3
Answers
Explanation:
The metal potassium and nonmetal chlorine will react together to form potassium chloride.
The formula for the cation potassium is K+. The anion chloride is Cl-. If we combine them to form a neutral compound we get:
Answer: A. KCl
The reaction mixture, at a certain temperature, contained concentrations of0.31 M of NH3, 0.85 M of N2 and 0.031 M of H2 when it reached equilibrium.Calculate Keq at this temperature.
Answers
ANSWER
Keq is 3798.42 or 3.798 x 10^3
EXPLANATION
Given that;
The concentration of NH3 is 0.31M
The concentration of N2 is 0.85M
The concentration of H2 is 0.031M
Follow the steps below to find the chemical equilibrium constant of the reaction
Step 1; Write the balanced equation of the reaction
[tex]\text{ N}_{2(g)}\text{ + 3H}_{2(g)}\text{ }\rightleftarrows\text{ 2NH}_{3(g)}[/tex]Step 2; Write the chemical equilibrium constant for the reaction
[tex]\begin{gathered} \text{ K}_{eq}\text{ = }\frac{K_f}{\text{ K}_b} \\ \text{ Where} \\ \text{ K}_f\text{ = }\lbrack NH_3\rbrack^2 \\ \text{ K}_b\text{ = }\lbrack N_2\rbrack\text{ }\lbrack H_2\rbrack^3 \end{gathered}[/tex]Step 3; Substitute the kf and kb into the formula above
[tex]\text{ K}_{eq}\text{ = }\frac{\lbrack NH_3\rbrack^2}{\lbrack H_2\rbrack^3\times\lbrack N_2\rbrack}[/tex]Recall, that
[NH3] = 0.31M
[H2] = 0.031M
[N2] = 0.85M
[tex]\begin{gathered} \text{ K}_{eq}\text{ = }\frac{(0.31)^2}{(0.85)\times\text{ \lparen0.031\rparen}^3} \\ \\ \text{ K}_{eq}\text{ = }\frac{\text{ 0.0961}}{0.85\text{ }\times\text{ 0.0000298}} \\ \\ \text{ K}_{eq}\text{ = }\frac{\text{ 0.0961}}{0.0000253} \\ \\ \text{ K}_{eq}\text{ = 3798.42 or 3.798 }\times\text{ 10}^3 \end{gathered}[/tex]Therefore, Keq is 3798.42 or 3.798 x 10^3
what compound is produced Be and Cl
Answers
Answer: Beryllium Chloride
I attached a picture of the questions I have. These are separate questions.
Answers
Electron configuration is defined as the description of where each electron is located, in terms of levels and sublevels around the nucleus. In this question, we have the following electron configuration:
1s2 2s2 2p6 3s2 3p6
This electron configuration has 8 electrons in its valence shell (the last number represents the shell number, in this case is 3) and according to the octet rule, this is a stabilized element, therefore it must be a Noble Gas
The period will be 3, since this is the valence shell
The group will be Noble gas, or group 18
Noble gas, usually, will not bond to other elements, since they are stabilized according to the octet rule
Help with this question, I’ve already got part a done I need part b and c
Answers
The number of moles of excess reagent left after the reaction is complete would be sodium phosphate and The mass of excess reagent that will remain unreacted is copper (II) nitrate.
The molar amounts of reactants and products that will result from a balanced chemical equation are shown. Rarely are reactants combined in the exact amount required in the actual world. Before the others, one reactant will be entirely depleted. The limiting reactant is the one that is consumed first in a reaction. The residual amount is deemed "in excess" once the other reactants have been partially consumed.
Calculate the volume of the product each reactant produced to identify the limiting reactant. The limiting reactant is the one that yields the smallest amount of product.
To know more about excess reagent visit : https://brainly.com/question/11848702
#SPJ9
Infer how resting membrane potential would be affected if the membrane were only permeable to potassium ions.
Option 1) If the cell were only permeable to potassium ions there would be a drastic change in membrane potential to a more positive value as potassium enters the cell
Option 2) If the cell were only permeable to potassium ions there would be a slight change in membrane potential to a more negative value as potassium exits the cell.
Option 3) If the cell were only permeable to potassium ions there would be a drastic change in membrane potential to a more negative value as potassium leaves the cell.
Answers
According to the research, the correct answer is Option 2. If the cell were only permeable to potassium ions there would be a slight change in membrane potential to a more negative value as potassium exits the cell.
What is resting membrane potential?It refers to the potential difference on either side of the membrane of a resting cell, that is, already repolarized where the channels are much more permeable to potassium than to sodium.
In this sense, it is determined by the ion concentration and membrane permeability where the inside of the cell has a more negative charge (potassium ions) relative to the outside (sodium ions).
Therefore, we can conclude that resting membrane potential causes the cell membrane inside to become negative because it has sodium ions towards the outside of the cell and potassium ions towards the inside, thus the correct answer is Option 2.
Learn more about resting membrane potential here: https://brainly.com/question/12530093
#SPJ1
Please solve this!! I won't be able totalk since I'm in school but just end it ifim not there and I'll give you a 5 starswhen I come back to look at it.
Answers
The first step to solve this question is to balance the given equation. To do it, make sure that the amount of every element is equal in both, reactants and products.
[tex]Mg_3N_2+3K_2O\rightarrow3MgO+2K_3N[/tex]Now, use the stoichiometric ratio between the coefficients of potassium oxide and potassium nitride:
[tex]14molK_2O\cdot\frac{1molMg_3N_2}{3molK_2O}=4.66molMg_3N_2[/tex]The answer is 4.66moles of magnessium nitride.
REACTION; C5H12 + 8O2 5CO2 + 6H2OWhen 35.5 L of C5H12 are consumed in this reaction what volume of CO2 can be produced in liters?
Answers
Using the STP (standard temperature and pressure), we can solve this problem. First, let's find the number of moles produce by 35.5 L of C5H12.
Remember that the standard temperature is 0 °C which is the same that 273 K (kelvin) and for pressure is 1 atm and the constant of ideal gas is 0.082 atm*L/(mol*K)
Let's use the formula of an ideal gas to find the number of moles:
[tex]\begin{gathered} PV=\text{nRT,} \\ n=\frac{PV}{RT}, \\ n=\frac{\text{1 atm }\cdot35.5\text{ L}}{0.082\frac{atm\cdot L}{mol\cdot K}\cdot273K}, \\ n(C_5H_{12})=1.58\text{6 moles} \end{gathered}[/tex]Now, using this number of moles, we can find the number of moles produce for CO2 and we can find its volume.
You can see that in the reaction 1 mol of C5H12 produces 5 moles of CO2, so the calculation to find the number of moles of CO2, would be:
[tex]1.586molC_5H_{12}\cdot\frac{5molCO_2}{1molC_5H_{12}_{}}=7.93molCO_2.[/tex]The next and final step is clear V (volume) from the initial formula and replaces the value of moles for CO2, like this:
[tex]\begin{gathered} V=\frac{nRT}{P}, \\ V=\frac{7.93\text{ mol}\cdot0.082\frac{atm\cdot L}{mol\cdot K}\cdot273K}{1\text{ atm}}, \\ V(CO_2)=177.52\text{ L.} \end{gathered}[/tex]So, 25,5 L of C5H12 will produce 177.52L of CO2.
What is the molarity of “Cl” in each solution? -0.200 M of NaCl-0.190 M of SrCl2-0.110 M of AlCl3
Answers
a) The molarity of Cl⁻ in 0.200 M of NaCl is 0.200 M.
a) The molarity of Cl⁻ in 0.190 M of SrCl₂ is 0.380 M.
c) The molarity of Cl⁻ in 0.110 M of AlCl₃ is 0.330 M.
a) 0.200 M of NaCl
The chemical reaction,
NaCl(aq) → Na⁺(aq) + Cl¯(aq)
From the above-balanced equation,
1 mole of Cl was created from 1 mole of NaCl
Therefore,
0.200 M NaCl will produce 0.200 M of Cl⁻
b) 0.190 M of SrCl₂
The chemical reaction:
SrCl₂(aq) → Sr²⁺ (aq) + 2Cl¯ (aq)
From the above-balanced equation,
2 moles of Cl were created from 1 mole of SrCl2.
Hence,
0.190 M of SrCl₂ will produce = 0.190 × 2 = 0.380 M of Cl⁻
c) 0.110 M of AlCl₃
The chemical reaction:
AlCl₃(aq) → Al³⁺ (aq) + 3Cl¯ (aq)
From the above-balanced equation,
3 moles of Cl were created from 1 mole of AlCl₃
Hence,
The molarity of Cl⁻ in 0.110 M of AlCl₃ is:
= 0.110 × 3 = 0.330 M
Learn more about molarity here:
brainly.com/question/26873446
#SPJ9
If the freezing point of a soda is – 4.5 ºC and you leave it in your car overnight when the temperature dips to 25 ºF, will the soda freeze? (Pay close attention to the units!)
Answers
Explanation:
We leave a soda in the car overnight and the temperature dips to 25 °F. To see if it will freeze we have to convert the °F to °C.
T(°C) = ( T(°F) - 32 ) *5/9
T(°C) = (25 - 32) *5/9
T(°C) = -3.9 °C
Since -3.9 °C is a temperature greater than the freezing point (-4.5 °C) the soda won't freeze.
Answer: The soda won't freeze.
The molar mass of NO2 is
46.01 g/mol.
What is the mass
of 3.45 moles NO2?
[ ? ] g NO₂
Answers
The mass of nitrogen dioxide present in 3.45 moles is equal to 158.73 g.
What is a mole?A mole can be defined as a scientific unit that is used to determine the huge number of quantities of molecules, atoms, ions, etc. The mass of the 1 mole of an element is called atomic mass and the mass of one mole of any compound is called molar mass.
The number of particles in 1 mole was found to be equal to 6.023 × 10 ²³ per mole which is known as Avogadro’s constant.
Given, the number of moles of nitrogen dioxide = 3.45 mol
The molar mass of NO₂ = 46.01 g/mol
It means that 1 mole of NO₂ has mass = 46.01 g
Then 3.45 moles of NO₂ will have mass = 3.45 × 46.01 = 158.73 g
Learn more about the mole, here:
brainly.com/question/26416088
#SPJ1
Recalling that a beaker of water is two dimensional, what is the three dimensional shape of the micelle?
Answers
The three dimensional shape of the micelle is sphere
micelle is a loosely bound aggregation of several hundreds of atoms, ions which are electrically charged atoms or molecules, which results in forming a colloidal particle. Micelles are basically aggregate or supramolecular assembly of surfactant of amphipathic lipids molecules dispersed in a liquid, forming a colloidal suspension which is also known to be associated with colloidal system.
Micelles are generally composed of hydrophobic and hydrophilic components that are assembled into nanosized spherical, ellipsoid, cylindrical, or uni lamellar structures. Usually the heads are hydrophilic in nature and the tail part is hydrophobic in nature. Therefore their structure in three dimension will be spherical.
To know more about micelle
https://brainly.com/question/28835340
#SPJ1
7. How many moles of NH3 can be produced from5.00 moles of H, according to the following equation?N2 + 3 H2 → 2 NH3a. 0.67 molb. 2.00 molc. 3.33 mold. 7.50 mol
Answers
Answer:
[tex]C\text{ : 3.33 mol}[/tex]Explanation:
Here, we want to get the number of moles of ammonia that could be produced from 5 moles of hydrogen
From the balanced equation of reaction, 3 moles of hydrogen produced 2 moles of ammonia
5 moles of hydrogen will produce x moles of ammonia
Mathematically:
[tex]\begin{gathered} 5\times2\text{ = 3}\times x \\ x\text{ = }\frac{10}{3} \\ x\text{ = 3.33 mol} \end{gathered}[/tex]The pressure exerted by 1.30 mol of gas in a 13.0 L container at 295 K is
Answers
Answer:
The pressure of the gas is 2.42atm.
Explanation:
The given information from the exercise is:
- Number of moles (n): 1.30 moles
- Volume (V): 13.0L
- Temperature (T): 295K
With the Ideal Gases Law formula we can calculate the pressure (P) of the gas, by replacing the values of n, V and T:
[tex]\begin{gathered} P*V=n*R*T \\ P*13.0L=1.30mol*0.082\frac{atm*L}{mol*K}*295K \\ P*13.0L=31.45atm*L \\ P=\frac{31.45atm*L}{13.0L} \\ P=2.42atm \end{gathered}[/tex]So, the pressure of the gas is 2.42atm.
I need to balance the equation __kciO3➡️__kcio4+__ KCIAnd How many moles of potassium chloride are produced from 3.90 moles of potassium chlorate ?
Answers
Explanation:
To balance the equation, we need to have the same number of atoms of each element on both sides of the equation.
So in this case:
4KCIO3 → 3KCIO4 + KCI
where:
reactants:
K - 4
Cl - 4
O - 12
Products:
K - 4
Cl - 4
O - 12
Potassium chloride is KCl and potassium chlorate is KClO3. The balanced reaction equation tells us that:
4 mol of KClO3 produces 1 mol of KCl, we know it because of the equation:
4 KCIO3 → 3 KCIO4 + 1 KCI
So we can do a rule of 3 to find out the quantity in moles produced when we have 3.90 moles of KClO3:
4 mol KClO3 --- 1 mol KCl
3.9 moles KClO3 --- x
4x = 1*3.9
x = 3.9/4
x = 0.975 moles
Answer: 4 KCIO3 → 3 KCIO4 + KCI and 0.975 moles.
A gas's volume decreases. Which of the following could most easily explain that?a. Its temperature increased.b. Its pressure increased.c. Its energy content increased.d. Its density decreased
Answers
This is an ideal gas problem, so let's see the relationships of volume with pressure and temperature:
- The relationship between volume and pressure is inversely proportional.
- The relationship between volume and temperature is directly proportional.
When the gas's volume decreases, the temperature would decrease too but the pressure would increases, so the answer would be B. Its pressure increased.
Based on the equation below, which of the following statements best describes what should be observed as the reaction takes place?Zn(s) + 2HCl(aq) to ZnCl2 (aq)+ H2(g)Solid zinc dissolvingBubbles forming from chlorine gas productionBubbles forming from hydrogen gas productionChlorine forming a solid precipitate
Answers
ANSWER
Bubbles forming from hydrogen gas production
EXPLANATION
Given that;
[tex]\text{ Zn}_{(s)}\text{ + 2HCl}_{(aq)}\rightarrow\text{ ZnCl}_{2(aq)}\text{ + H}_{2(g)}[/tex]One of the method used in the preparation of hydrogen gas is the reaction of metals with acid
The above reaction is one of the method of producing hydrogen gas
When Zn reacts with HCl, bubbles formed which is as a result of hydrogen production.
Therefore, the correct answer is Bubbles forming from hydrogen gas production
I need help with this. What do I add to make the after reaction
Answers
According to the given reaction, one molecule of H2 reacts with one molecule of C2H4 to produce one molecule of C2H6.
In the reactants, there are 7 molecules of C2H4 and 3 molecules of H2, it means that 3 molecules of C2H4 react with the 3 molecules of H2 to form 3 molecules of C2H6, and 4 molecules of C2H4 will remain.
It means that you have to add 3 molecules of C2H6 and 4 molecules of C2H4.
The charge of an election is
Answers
The charge of an electron is negative (-1).
A volume of 56.0 mL of He at STP. a. How much weight?
Answers
We already know that number of moles is 0.0025 mol.
So if we know the volume of the gas at STP and the number of moles we can get the mass of the gas.
V = 0.056 L
1 mol = 22.4 L
Molar mass of He = 4.0026 g/mol
So to calculate the weight we need number of moles and molar mass.
weight = 0.0025 mol x 4.0026 g/mol
= 0.01 g
The equation for the synthesis of ammonia is N2 + 3 H2 -> 2 NH3. How many moles of H2 I needed to produce 6 mol of NH3? A. 4 B. 6 C. 8 D. 9
Answers
The equation for the synthesis of ammonia is:
N₂ + 3 H₂ ---> 2 NH₃
We have to find the number of moles of H₂ needed to produce 6 mol of NH₃. To do that we will use the equation that we were given.
We can read the equation of the reaction as a recipe. When 1 mol of N₂ reacts with 3 moles of H₂, 2 moles of NH₃ are produced. So the relationship between H₂ and NH₃ is that 3 moles of H₂ are needed to produce 2 moles of NH₃. We wiill use that relationship to solve the problem.
6 moles of NH₃ * 3 moles of H₂/(2 moles of NH₃) = 9 moles of H₂
Answer: D) 9 moles of H₂ are needed.
In the equation: I2 + 5Cl2 + 6 H2O —>2HIO3 + 10HCL A. Has iodine been oxidized or has it been reduced B. Has chlorine been oxidized or has it been reduced
Answers
Answer
A - Iodine is being oxidized
B - Cl2 is being reduced
Explanation
[tex]I_{2(s)}^0\text{ + 5Cl}_{2(g)}^0\text{ + 6H}_2O_{(l)}\rightarrow2HI^VO_{3(aq)}\text{ + HCl}_{(aq)}^{-I}[/tex]Mateo has sprayed luminol on a bed spread but sees no evidence of any substances. What mistake is he MOST likely making?
A.
He is spraying too much luminol.
B.
He is diluting the substances with luminol.
C.
He is leaving the lights on in the room.
D.
He is making it too dark in the room.
Answers
Answer:b
Explanation:
because i said so
3Ca + 2AICI3 —> 3CaCI2 + 2AIIf you react 100, grams of aluminum chloride, AICI3, with excess calcium, how many grams of calcium chloride,CaCI2, are produced ?
Answers
Firstly, we need to convert the 100 g of AlCl₃ to number of moles, using:
[tex]M_{AlCl_3}=\frac{m_{AlCl_3}}{n_{AlCl_{3}}}[/tex]The molar weight of AlCl₃ is calculating consulting the atomic weights:
[tex]\begin{gathered} M_{AlCl_3}=1\cdot M_{Al}+3\cdot M_{Cl} \\ M_{AlCl_3}=(1\cdot26.982+3\cdot35.453)g/mol \\ M_{AlCl_3}=(26.982+106.359)g/mol \\ M_{AlCl_3}=133.341g/mol \end{gathered}[/tex]Thus:
[tex]\begin{gathered} M_{AlCl_3}=\frac{m_{AlCl_3}}{n_{AlCl_3}} \\ n_{AlCl_3}=\frac{m_{AlCl_3}}{M_{AlCl_3}}=\frac{100g}{133.341g/mol}=0.749957\ldots mol \end{gathered}[/tex]For each 2 moles of AlCl₃, we produce 3 moles of CaCl₂. So:
2 mol AlCl₃ --- 3 mol CaCl₂
0.749957... mol AlCl₃ --- x
[tex]\begin{gathered} \frac{2}{0.749957\ldots mol}=\frac{3}{x} \\ 2x=2.24987\ldots mol \\ x=1.12494\ldots mol \end{gathered}[/tex]Now, we convert back to grams, but now we need the molar weight of CaCl₂:
[tex]\begin{gathered} M_{CaCl_2}=1\cdot M_{Ca}+2\cdot M_{Cl} \\ M_{CaCl_2}=(1\cdot40.078+2\cdot35.453)g/mol \\ M_{CaCl_2}=(40.078+70.906)g/mol \\ M_{CaCl_2}=110.984g/mol \end{gathered}[/tex]Thus:
[tex]\begin{gathered} M_{CaCl_{2}}=\frac{m_{CaCl_2}}{n_{CaCl_{2}}} \\ m_{CaCl_2}=M_{CaCl_2}\cdot n_{CaCl_2}=110.984g/mol\cdot1.12494\ldots mol=124.850\ldots g\approx125g \end{gathered}[/tex]So, 100 g of AlCl₃ will produce approximately 125 g of CaCl₂.
explain which mgcl2 and alcl3 is stronger bonding?
Answers
AlCl3 has a higher charge density which makes the polarization to be high. Therefore AlCl3 has a higher covalent character (stronger bonding).
Covalent character and cationic size are indirectly related to each other. When the size of a cation is smaller, the covalent character would be higher.
In MgCl2, the cation is Mg^2+ and in AlCl3, the cation is Al^3+. The size of Mg^2+ is larger when compared to the size of Al^3+. That is why the covalent character of AlCl3 is larger.
I got 0.009 and I was wondering if that is right
Answers
Explanation:
The equation of formation of NO₂
1/2 N₂ (g) + O₂ (g) <----> NO₂ (g)
The equilibrium for the above reaction can be calculated using this equation:
Kc = [NO₂]/([N₂]^(1/2)*[O₂]) where the concentrations are the equilibrium concentrations.
According to the graph the concentration in the equilibrium of each compound is:
[NO₂] = 6 mol/L
[N₂] = 10 mol/L
[O₂] = 20 mol/L
If we replace these values in the given equation:
Kc = [NO₂]/([N₂]^(1/2)*[O₂])
Kc = (6 mol/L)/[(10 mol/L)^(1/2)*(20 mol/L)]
Kc = 0.095
Answer: Kc = 0.095
this is for my final review please help also any advice is helpful
Answers
Answer:
[tex]Saturated[/tex]Explanation:
Here, we want to get the given definition
We have the solvent taking in as much solute as it can take
That is what we call a saturated solution
It simply means the amount of solute in the solvent is at the maximum
PLEASE HELP - 100 PTS AND BRAINLIEST
Indicate the number of protons, neutrons, and electrons in isolated ions having the following nuclear symbols:
a) 19F1- has ? protons, ? neutrons, and ? electrons.
b) 24Mg2+ has ? protons, ? neutrons, and ? electrons.
c) 56Fe3+ has ? protons, ? neutrons, and ? electrons.
Answers
The number of protons, neutrons, and electrons in isolated ions having the following nuclear symbols:
a) ¹⁹F⁻ has 9 protons , 9 neutrons , 10 electrons
b) ²⁴Mg²⁺ has 12 protons , 12 neutrons , 10 electrons
c) ⁵⁶Fe³⁺ has 26 protons , 26 neutrons , 23 electrons
Atomic number represent the no. of protons. number of protons is equal to number of electrons in neutral atom. neutrons is the difference between mass number and atomic number.
a) Atomic number of fluorine is 9. for neutral F atom has 9 protons , 9 neutrons , 9 electrons. In ¹⁹F⁻ , -1 represents one extra electron.
¹⁹F⁻ has 9 protons , 9 neutrons , 10 electrons
b) Atomic number of Magnesium is 12. In ²⁴Mg²⁺ , 2+ represents 2 electrons are removed.
²⁴Mg²⁺ has 12 protons , 12 neutrons , 10 electrons
c) Atomic number of iron is 26.
⁵⁶Fe³⁺ has 26 protons , 26 neutrons , 23 electrons
Thus, The number of protons, neutrons, and electrons in isolated ions having the following nuclear symbols:
a) ¹⁹F⁻ has 9 protons , 9 neutrons , 10 electrons
b) ²⁴Mg²⁺ has 12 protons , 12 neutrons , 10 electrons
c) ⁵⁶Fe³⁺ has 26 protons , 26 neutrons , 23 electrons
To learn more about electrons protons neutrons here
https://brainly.com/question/11506972
#SPJ1
Answer question number 11. The question is in the image.
Answers
Answer
Explanation
Ethane has a molecular formula of C₂H₆. Its structural formula is CH₃-CH₃ or
What's the volume of 0.2 mol of nitrogen at 20oC and 0.7 atm?
Answers
Explanation:
We have to determine the volume of 0.2 mol of nitrogen at 20 °C and 0.7 atm. The ideal gas law describes this situation.
P * V = n * R * T
Where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant and T is the temperature.
We already know these some of these values.
P = 0.7 atm
n = 0.2 mol
R = 0.082 atm * L/(mol*K)
T = 20 ° C = 293.15 K
We can use the ideal gas law to determine the volume of the sample.
P * V = n * R * T
V = n * R * T/P
V = 0.2 mol * 0.082 atm*L/(mol*K) * 293.15 K/(0.7 atm)
V = 6.86 L
Answer: The volume is 6.86 L.