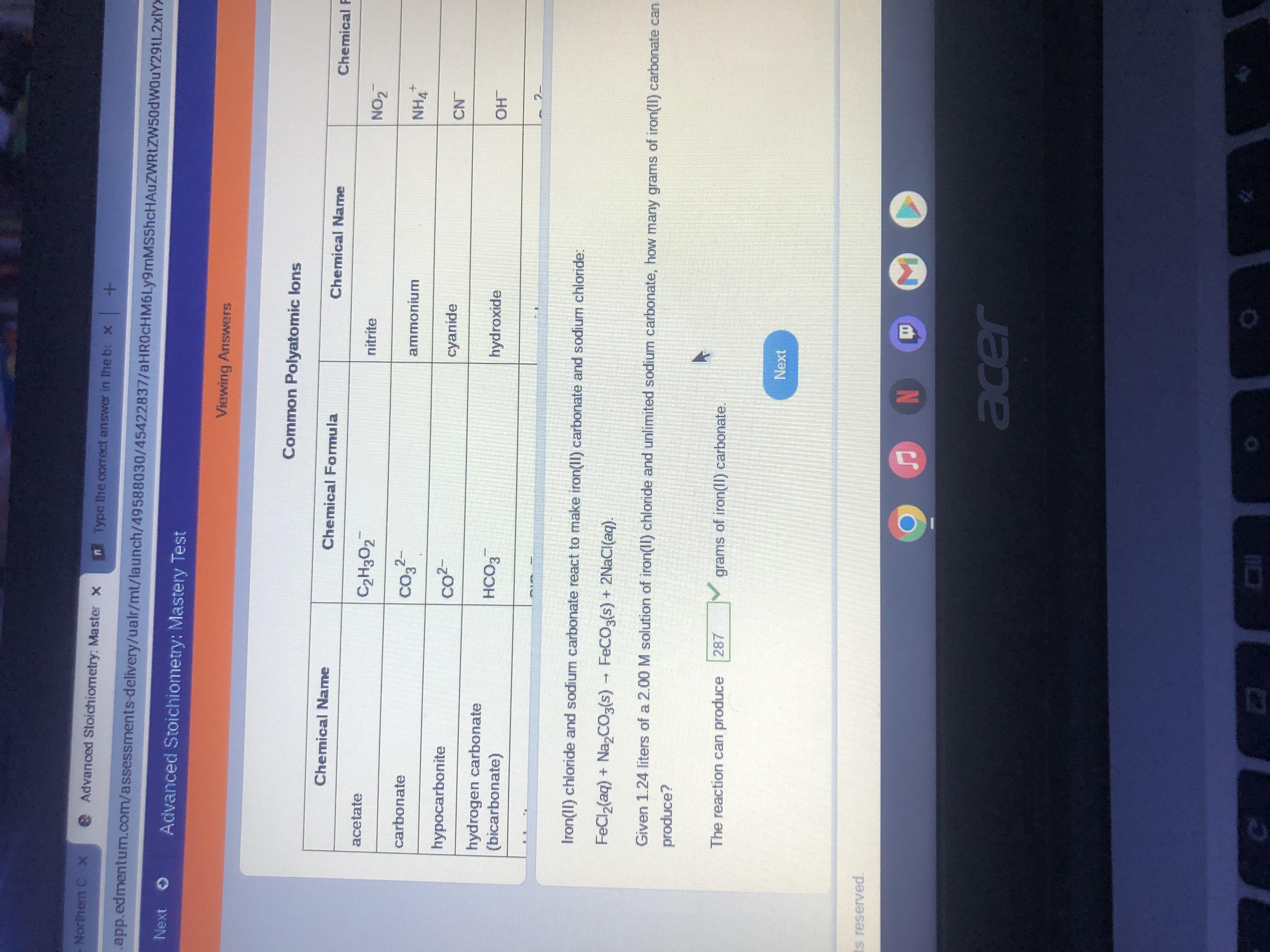
Type the correct answer in the box. Express your answer to three significant figures. Iron(II) chloride and sodium carbonate react to make iron(II) carbonate and sodium chloride: FeCl2(aq) + Na2CO3(s) → FeCO3(s) + 2NaCl(aq). Given 1.24 liters of a 2.00 M solution of iron(II) chloride and unlimited sodium carbonate, how many grams of iron(II) carbonate can the reaction produce? The reaction can produce grams of iron(II) carbonate.
Answers
Answer:
Explanation:
To solve this problem we have to find the moles of iron(II) chloride that react. Using the chemical equation, we can kknow moles FeCl2 = Moles FeCO3. Thus, we can find the moles of FeCO3. Converting these moles to grams using its molar mass -Molar mass FeCO3: 115.854g/mol-
Moles FeCl2 = Moles FeCO3:
1.24L * (2.00mol / L) = 2.48 moles FeCl2
Mass FeCO3:
2.48mol * (115.854g / mol) =
The reaction can produce 287 grams of iron(II) carbonateAnswer:
its 287
Explanation:
i got it right on edm
Related Questions
A rocket launches because of __________.
A. an opposite reaction
B. it's large mass
C. its gravity
D. air resistance
Answers
Answer:
an opposite reaction
Earth's gravity is still pulling down on the rocket. When a rocket burns propellants and pushes out exhaust, that creates an upward force called thrust. To launch, the rocket needs enough propellants so that the thrust pushing the rocket up is greater than the force of gravity pulling the rocket down.
Help for brainliest
Answers
Answer:
those things that looks like cristals
In 2Fe+3Cl2=2FeCl3, how much iron (Fe) is needed to produce 4 moles of FeCl3?
In 2Fe+3Cl2=2FeCl3, how much FeCl3 is produced when 5 moles of Fe react in excess chlorine?
Answers
Answer:
4 mol Fe
5 mol FeCl₃
Explanation:
Step 1: Write the balanced equation
2 Fe + 3 Cl₂ ⇒ 2 FeCl₃
Step 2: Calculate how much iron (Fe) is needed to produce 4 moles of FeCl₃
According to the balanced equation, the molar ratio of Fe to FeCl₃ is 2:2.
4 mol FeCl₃ × 2 mol Fe/2 mol FeCl₃ = 4 mol Fe
Step 3: Calculate how much FeCl₃ is produced when 5 moles of Fe react in excess chlorine
According to the balanced equation, the molar ratio of Fe to FeCl₃ is 2:2.
5 mol Fe × 2 mol FeCl₃/2 mol Fe = 5 mol FeCl₃
A small ferret comes to help Roxie with the rock that is blocking her den. They push with a total force of 90 N moving the boulder a distance of 5m. How much work do they do?
Answers
Answer:
Work = 450 J
Explanation:
Force = 90N
Distance = 5m
Work = ?
The relationship between these quantities is given by the equation:
Work = Force * Distance
Work = 90 * 5
Work = 450 J
Consider the statement:
“The following report presents the results of our laboratory tests, which support the conclusion that our new treatment for acne is safe and effective with minimal possible side effects.”
What questions should a person ask themselves to determine if this scientific study is reliable?
What does the acne medicine look like?
What does the acne medicine smell like?
Were laboratory tests conducted by the same company that produces the acne medicine?
Were laboratory tests conducted in a laboratory with more than 100 employees?
Answers
The question the person should ask him/herself to probably check for authenticity of the drugs is "Were laboratory tests conducted by the same company that produces the ac[tex]\cap[/tex]e medicine?". Option C. This is further explained below.
What is a scientific study?Generally, scientific study is simply defined as a method that includes making conjectures and deriving answers as logical implications from the hypotheses.
In conclusion, Were laboratory tests conducted by the same company that produces the ac[tex]\cap[/tex]e medicine should be questioned as medical companies should look to other labs for authenticity confirmation.
Read more about medicine
https://brainly.com/question/5591489
#SPJ2
36 g of KOH was dissolved in 800 mL of water. What is the
molality of the solution? (Molar mass of KOH = 56 g/mol,
density of water = 1.00 g/mL) ___ m.
Answers
Answer: 0.8 m
Explanation:
A rarefraction is generated when particles move
A:together
B.closer
C.under
D.apart
Answers
How many joules of heat are needed to raise the temperature of 30.0 g of aluminum from 22°C to 80°C, if the specific heat of aluminum is 0.90 J/g°C?
Answers
Answer: There are 1566 joules of heat needed to raise the temperature of 30.0 g of aluminum from 22°C to 80°C, if the specific heat of aluminum is 0.90 J/g°C.
Explanation:
Given: Mass = 30.0 g
Specific heat = [tex]0.90 J/g^{o}C[/tex]
[tex]T_{1} = 22^{o}C[/tex]
[tex]T_{2} = 80^{o}C[/tex]
Formula used to calculate the heat energy requires is as follows.
[tex]q = m \times C \times (T_{2} - T_{1})[/tex]
where,
q = heat energy
m = mass of substance
C = specific heat capacity of substance
[tex]T_{1}[/tex] = initial temperature
[tex]T_{2}[/tex] = final temperature
Substitute the values into above formula as follows.
[tex]q = m \times C \times (T_{2} - T_{1})\\= 30.0 g \times 0.90 J/g^{o}C \times (80 - 22)^{o}C\\= 1566 J[/tex]
Thus, we can conclude that there are 1566 joules of heat needed to raise the temperature of 30.0 g of aluminum from 22°C to 80°C, if the specific heat of aluminum is 0.90 J/g°C.
NEED HELP ASAP 20 POINTS !!!!!What does a subscript in a chemical formula tell you? For example the 3 in Rb3PO4 the number of atoms of the element symbol it follows the number of atoms of the element symbol it is in front of the number of molecules
Answers
Answer:
The answer should be A
Explanation:
because the definition of a subscript is that they indicate the number of atoms of an element present in am molecule or formula unit.
Answer:
The first choice. The rule is go left. Those subscript numbers always talk about the element to the left of them.
Explanation:
The straight forward answer is the first one.
You should always answer that when reading the chemical make up of molecules. Let's look at some examples.
C8 H18
Consider the 8. It must be describing how many carbons there are. If it was meant for the hydrogen, then what does the 18 mean? You wouldn't know.
Now look at something a lot more complicated.
Mg3(PO4)2
Use the going left rule. That satisfies Mg doesn't it? There are 3 of them and 3 is correct.
The two at the end is also straightforward if you think about it. It must be telling you how many molecules are in the brackets. If that were not so, then you wouldn't be able to tell what it did. So there are 2 PO4
But you still have the 4 to deal with. Use the going left rule again. 4 must be telling you how many oxygens you have inside the brackets. There are 4 of them.
The greenhouse gas released into the atmosphere as a result of livestock digesting their food is called carbon dioxide
True
False
Answers
Answer:
False
Explanation:
Cows and other livestock release methane, which is a gas like Carbon Dioxide, but not the same thing, even though it does contribute into Carbon Dioxide.
The balanced chemical equation for the reaction between sodium chloride and silver nitrate is:
NaCl ( aq ) + AgNO3 ( aq ) AgCl ( s ) + NaNO3 ( aq )
We can interpret this to mean:
1 mole of sodium chloride and mole(s) of silver nitrate
React to produce:
mole(s) of silver chloride and mole(s) of sodium nitrate
Answers
Answer:
We can Interprete it as 1mole of Sodium Chloride and 1mole of Silver Nitrate React to Produce
1Mole of Silver Chloride and 1Mole of Sodium Nitrate
Answer:NaCL
Explanation:Edg
The valences of metal x,y and z are 1,2 and 3 respectively. What are the formulae of their;a) hydroxides, b) sulphates, c) hydrogen, d) carbonates, e) nitrates, f) phosphates
Answers
Answer:
See answer below
Explanation:
AS we know that the valence for those metals X, Y, and Z are 1, 2 and 3, we can determine the formula of each compound.
1. Hydroxides.
An hydroxide is formed when an oxyde of a metal reacts with water. When this happens, the general molecular formula is:
Meₐ(OH)ₙ
Where:
a: valence or charge of the hydroxide (Which is -1)
n: valence of the metal.
Following this, the formula for X, Y and Z would be:
XOH
Y(OH)₂
Z(OH)₃
2. Sulphates
Sulphates follow a similar rule of hydroxide in the general molecular formula, but instead of having a charge of -1, it has a charge of -2 so:
Mₐ(SO₄)ₙ
So, following the rule:
X₂SO₄
Y₂(SO₄)₂ ------> YSO₄
Z₂(SO₄)₃
3. Hydrogens
Following the same rule as the previous, hydrogens works with a charge of -1, so:
MₐHₙ
Then:
XH
YH₂
ZH₃
4. Carbonates.
This follows the same rule as sulphates, with the same charge so:
Mₐ(CO₃)ₙ
Then:
X₂CO₃
YCO₃
Z₂(CO₃)₃
5. Nitrates
Follow the same rule as the hydroxides, with the same charge of -1.
Mₐ(NO₃)ₙ
Then:
XNO₃
Y(NO₃)₂
Z(NO₃)₂
6. Phosphates
In the case of phosphates, these have a charge of -3 so:
Mₐ(PO₄)ₙ
Then:
X₃PO₄
Y₃(PO₄)₂
Z₃(PO₄)₃ ----> ZPO₄
Hope this helps
The speed you read from your speedometer is your ____________________. *
average speed
direction
instantaneous speed
distance
Answers
Answer:
it is your instantaneous speed
Explanation:
What happens during a lunar eclipse?
A
Earth passes between the sun and the moon.
B
The moon passes between the sun and Earth.
C
The sun passes between Earth and the moon.
D
Earth, the sun, and the moon are at right angles in space.
Answers
Answer:
A.EARTH PASSES BETWEEN THE SUN AND THE MOONExplanation:
When Earth passes directly between Sun and Moon, its shadow creates a lunar eclipse.
pabrainliest po plss:)
Convert 444 cal to joules
Answers
Answer: 1
Decimal Answer: 1.858
Which best describes nuclear fusion? O A nucleus spontaneously splits and absorbs energy O Two nuclei spontaneously combine and absorb energy. O A nucleus collides with a neutron and splits, releasing energy. O Nuclei combine to form a heavier nucleus, releasing energy.
Answers
Answer: Nuclei combine to form a heavier nucleus, releasing energy.
Explanation: e.g two deuterium nucleus (Hydrogen-2 isotopes) forms an He nucleus and energy is released.
Nuclear fusion is when nuclei combine to form heavier nuclei, releasing energy.
What is Nuclear Fusion?Nuclear fusion is a reaction in which two or more atomic nuclei are combined to form one or more different atomic nuclei and subatomic particles (neutrons or protons). The difference in mass between the reactants and products is manifested as either the release or absorption of energy.
The release of energy with the fusion of light elements is due to the interplay of two opposing forces: the nuclear force, a manifestation of the strong interaction, which holds protons and neutrons tightly together in the atomic nucleus; and the Coulomb force, which causes positively charged protons in the nucleus to repel each other.
Nuclear fusion is also observed in stars.
To learn more about, fusion:
https://brainly.com/question/16556922
#SPJ5
13) Which "conversion tool" below would you use to complete this
conversion?
Answers
Use the dichotomous key to identify these plant leaves.
Leaves from which plant are shown?
pine
catalpa
dogwood
maple
Answers
Answer:
catalpa
Explanation:
i took the test
How many grams would be in 1.752 x10^26 formula units of Ca(No3)2
Answers
Answer:
47730 grams.
Explanation:
First we convert 1.752x10²⁶ formula units of Ca(NO₃)₂ into moles, using Avogadro's number:
1.752x10²⁶ formula units ÷ 6.023x10²³ formula units/mol = 290.88 molThen we convert 290.88 moles of Ca(NO₃)₂ into grams, using the molar mass of Ca(NO₃)₂:
290.88 mol * 164.088 g/mol = 47730 g74g of Ca(OH)2 (74 g/mol) in 340 ml solution
Answers
Answer:
This question is asking to calculate the molarity of the solution based on the information provided.
The answer is 2.94M
Explanation:
Molarity of Ca(OH)2 solution = number of moles (n) ÷ volume (V)
Using the formula below to calculate number of moles of Ca(OH)2.
mole = mass/molar mass
Molar mass of Ca(OH)2 = 74 g/mol
mole = 74g/74g/mol
mole = 1mol
Volume of Ca(OH)2 solution = 340ml = 340/1000 = 0.340 L
Molarity = 1/0.340
Molarity = 2.94M
PLEASE HELP.
What does the atomic number of an element tell us?
A) the number of neutrons in an atom of an element
B) the number of protons in an atom of an element
C) the number of protons, electrons, and neutrons in an element
Answers
A solution is any mixture of two or more substances.
TRUE or
FALSE
Answers
Answer:
true
Explanation:
H2O2 (yield) H2O + O2
If 650 grams of hydrogen peroxide is allowed to decompose, how much water and
oxygen will be created? If 255 grams of oxygen are collected, what is the percent
yield?
Answers
2 H2O2 = 2 H2O + O2
What is described by the frequency of a wave?
A. The speed the wave is traveling through space
B. The number of waves that pass a point in 1 second
C. The height of the wave from trough to peak
D. The distance from one peak to the next peak
Answers
Taking into account the definition of frecuency, the statement "The number of waves that pass a point in 1 second" describes the frequency of a wave.
Definition of frequencyFrequency is the number of vibrations that occur in a unit of time. Its unit is s⁻¹ or hertz (Hz).
In other words, the frequency in wave phenomena, such as sound, electromagnetic waves (such as radio or light), electrical signals or other waves, expresses the number of times the phenomenon is repeated per unit of time. For example, if a wave repeats ten times per second, it means that it has a frequency of ten cycles per second.
SummaryFinally, the statement "The number of waves that pass a point in 1 second" describes the frequency of a wave.
Learn more about frequency:
https://brainly.com/question/1247008
https://brainly.com/question/12990032
#SPJ1
In the buffer solution CH3COsH(aq) -> H3O+(aq) + CH3CO2-(aq)
A. H3O+ is an acid, and CH3CO2H is its conjugate base.
B. CH3CO2H is an acid, and CH3CO2- is its conjugate base.
C. H3O+ is an acid, and CH3CO2- is its conjugate base.
D. CH3CO2H is a base, and H3O+ is its conjugate acid
Answers
In the buffer solution CH₃COOH (aq) ---> H₃O⁺(aq) + CH₃CO₂⁻(aq):
CH₃CO₂H is a base, and H₃O⁺ is its conjugate acid.
Option D is correct.
What is an acid?An acid is described as a molecule or ion capable of either donating a proton, known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid.
From the buffer solution above, H₃O⁺ is the conjugate acid because it donates a proton (H⁺) to a base CH₃CO₂H.
In conclusion, A conjugate acid, within the Brønsted–Lowry acid–base theory, is described as a chemical compound formed when an acid donates a proton (H⁺) to a base.
Learn more about acid at: https://brainly.com/question/25148363
#SPJ1
Its is the change in energyof a neutral atom when an electron to the atom to form negativeion, thus neutral atom gain electon.What property of element is being described
Answers
Answer:
Electron affinity.
Explanation:
An atom can be defined as the smallest unit comprising of matter that forms all chemical elements. Thus, atoms are basically the building blocks of matters and as such determines or defines the structure of a chemical element.
Generally, atoms are typically made up of three distinct particles and these are protons, neutrons and electrons.
Electron affinity can be defined as the ability of an atom of a chemical element to accept or accommodate an electron.
This ultimately implies that, electron affinity is the change in energy of a neutral atom of a chemical element when an electron is added to the atom to form negative ion, thus, a neutral atom gain electron.
In Chemistry, electrons can be defined as subatomic particles that are negatively charged and as such has a magnitude of -1.
At a high concentration do you have more or less particles per unit volume
Answers
Answer:
More particles per unit volume
Explanation:
Concentration means the amount of solute in a solution. Now, the amount of solute also means the number of particles of solute present in a solution.
Hence, when we use the term "high concentration", we imply that the amount of solute present or the number of particles present in a solution is high.
Thus, at high concentration, there are more solute particles than solvent particles in a solution.
a concentrated solution of sulfuric acid, H2SO4, has a concentration of 18.0 M. How many mL of the concentrated acid would be required to make 250. mL of a 1.00 MH2SO4 solution?
Answers
Answer: A 13.88 mL of the concentrated acid would be required to make 250. mL of a 1.00 M [tex]H_{2}SO_{4}[/tex] solution.
Explanation:
Given: [tex]M_{1}[/tex] = 18.0 M, [tex]V_{1}[/tex] = ?
[tex]M_{2}[/tex] = 1.00 M, [tex]V_{2}[/tex] = 250 mL
Formula used to calculate the volume of concentrated acid is as follows.
[tex]M_{1}V_{1} = M_{2}V_{2}[/tex]
Substitute the values into above formula as follows.
[tex]M_{1}V_{1} = M_{2}V_{2}\\18.0 M \times V_{1} = 1.00 M \times 250 mL\\V_{1} = \frac{1.00 M \times 250 mL}{18.0 M}\\= 13.88 mL[/tex]
Thus, we can conclude that 13.88 mL of the concentrated acid would be required to make 250. mL of a 1.00 M [tex]H_{2}SO_{4}[/tex] solution.
How many grams of H2 are needed to completely react within 50g of N2?
Answers
Answer: 10.7 grams
Therefore, 10.7 grams of Hydrogen are necessary to fully react with 50.0 grams of Nitrogen.
What is the pH of a 0.21 M solution of LiOH?
Answers
Answer:
13.32
Explanation:
pOH=-log(0.21)=0.68
pH=14-pOH=14-0.68=13.32