Answers
Explanation:
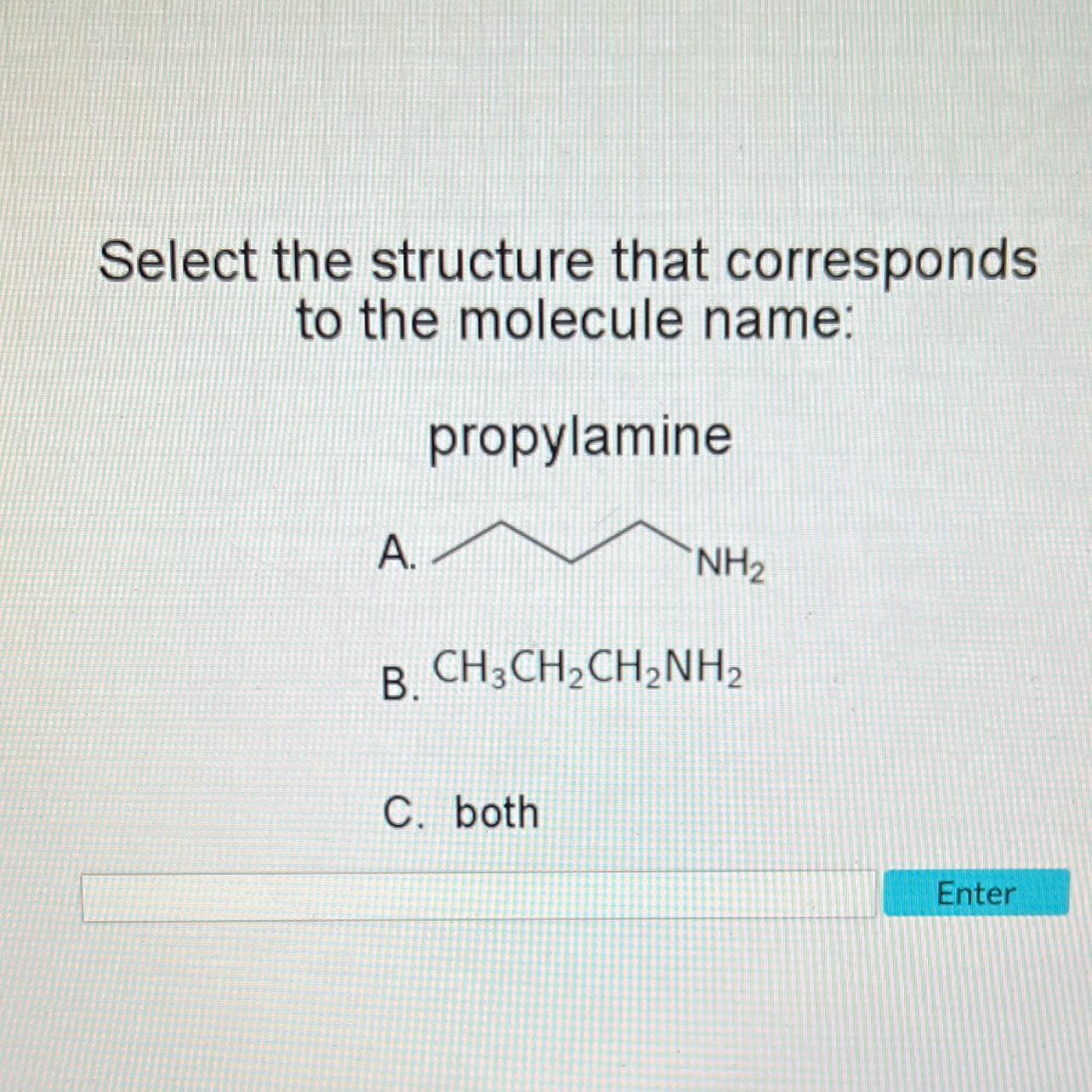
Propylamine is characterized by 3 carbons, 9 hydrogens, and 1 nitrogen.
According to the given picture, B is the correct answer.
Answer:
B is the correct answer.
Related Questions
true or false does photosynthesis takes place in all animal cells
Answers
Answer:
photosynthesis does not take place in animals
Explanation:
it doesn't because animals do not have plant cells
Question
Write and balance the half-reaction for the formation of perchlorate CIO from hypochlorite CIO in the presence of an aqueous acid.
Answers
ClO- + 3H2O--> ClO4- + 6H+ + 6e- is balance equation reaction
Stoichiometric coefficients for the reactants and products are necessary to balance chemical equations. Because a chemical equation must adhere to the principles of mass conservation and constant ratios, the same amount of atoms of each element must be present on two of the reactant and product sides of the equation.
The reactants and products of a chemical reaction are symbolically expressed in chemical equations using the appropriate chemical formulas. The portion of the chemical equation that is on the reactant side is to the left of the sign "," and to the right of the arrow symbol, respectively.
To know more about balance equation visit : brainly.com/question/28949107
#SPJ9
Which solution has a hydronium ion concentration of 1.00 x 10-5 M?A Solution with a PH of 4, 7, 3, or 5
Answers
Answer
A solution with pH of 5.
Explanation
Given:
[H₃O⁺] = 1.00 x 10⁻⁵ M
To know the solution that has a hydronium ion concentration, [H₃O⁺] of 1.00 x 10⁻⁵ M, you need to calculate the pH of the solution using the formula given below:
[tex]pH=-log\left[H₃O⁺\right][/tex]Put [H₃O⁺] = 1.00 x 10⁻⁵ M into the pH formula:fof
[tex]\begin{gathered} pH=-log(1.00\times10⁻⁵M) \\ \\ pH=-(-5) \\ \\ pH=5 \end{gathered}[/tex]PLEASE HELP - 100 PTS AND BRAINLIEST
Indicate the number of protons, neutrons, and electrons in isolated ions having the following nuclear symbols:
a) 19F1- has ? protons, ? neutrons, and ? electrons.
b) 24Mg2+ has ? protons, ? neutrons, and ? electrons.
c) 56Fe3+ has ? protons, ? neutrons, and ? electrons.
Answers
The number of protons, neutrons, and electrons in isolated ions having the following nuclear symbols:
a) ¹⁹F⁻ has 9 protons 10 neutrons 10 electrons
b) ²⁴Mg²⁺ has 12 protons 12 neutrons 10 electrons
c) ⁵⁶Fe³⁺ has 26 protons 30 neutrons 23 electrons
a) ¹⁹F⁻
The atomic no. of F is 9 . atomic number shows the no. of protons. the no. of protons is 9. number of protons = no. electrons for neutral atom but here -1 shows one extra electron. number of electrons is 10. neutrons is the difference in mass no. and the atomic number. number of neutrons = 19 - 9 = 10
¹⁹F⁻ has 9 protons 10 neutrons 10 electrons
b) ²⁴Mg²⁺
The atomic no. is 12 . number of protons is 12. +2 represents 2 electrons are removed. number of electrons is 10. neutrons = mass no. - atomic no. number of neutrons = 30
²⁴Mg²⁺ has 12 protons 12 neutrons 10 electrons
c) ⁵⁶Fe³⁺
The atomic number is 26. number of protons = 26. +3 means three electrons are removed. number of electrons are 23. neutrons = 56 - 26 = 30.
⁵⁶Fe³⁺ has 26 protons 30 neutrons 23 electrons
Thus, The number of protons, neutrons, and electrons in isolated ions having the following nuclear symbols:
a) ¹⁹F⁻ has 9 protons 10 neutrons 10 electrons
b) ²⁴Mg²⁺ has 12 protons 12 neutrons 10 electrons
c) ⁵⁶Fe³⁺ has 26 protons 30 neutrons 23 electrons
To learn more about protons neutrons electrons here
https://brainly.com/question/13131235
#SPJ1
How many inner, outer, and valence electrons are present in Fe?
Answers
Valence electrons are present in Fe is 8 and inner is 20 and outer is 8
A valence electron is the outer shell associated with an atom and that can participate in the formation of a chemical bond if the outer shell is not closed and in iron the atomic number of iron is 26 and hence, the valence electrons that are present in iron are 8 the outer electrons in iron are 8. The inner electrons in iron are 20
Know more about inner and outer electron
https://brainly.com/question/14655467
#SPJ1
If the students actually collected 145 mL of gas (.145L), what is the % yield for the experiment?
Answers
In order to calculate the percent yield of a reaction we will use:
%yield = (actual yield/theoretical yield) * 100
%yield = (0.145/0.105) * 100
%yield = 138%
Answer:
See attached worksheet
Explanation:
As the worksheet explains, the fiorst step is to have a balanced equation of the prioction of hydrogen gas from magnesium and hydrochloric acid.
2Mg + 2HCl ⇒ H2 + 2MgCl
Then convert the mass of manesium into moles Mg (0.045 moles Mg)
Since the balanced equation promises us that we'll get 1 mole of H2 for every 2 moles of Mg, we can predict that the 0.045 moles of Mg will be enough to make (0.045 moles Mg)*(1 mole H2/2 moles Mg) = 0.0225 moles of H2.
At 22.4 l/mole at STP, this means the gas volume of H2 is (0.0225 moles)*(22.4L/mole) = 0.504 L of H2 at STP.
Since the lab is not at STP, the above volume must be adjected for actual lab conditions. Use the combined gas law:
P1V1/T1 = P2V2/T2
where P is pressure, V is volume, and T is temperature, in Kelven. (add 273.15 to C for Kelvin). The subscripts 1 and 2 represent starting (1) and final (2) conditions.
Stand pressure in kPa is 101.3 kPa.
We want V2, so rearrange the equation:
V2 = V1(T2/T1)(P1/P2)
Note that I've arranged the tempaerature and pressure values as ratios. It helps guide the process for cancelling units and understanding the impact of changes in these values.
See the worksheet for more details. V2 is calculated to be 0.542L (542ml) of H2 under the lab conditions.
Percent theorectical yield is the amount obtained divided by the amount expected:
(145ml)/(542ml) = 26.8% theorectical yield.
Suppose a new element has been made. Chemical tests show it is an alkaline earth metal. a) Predict how many electrons will be in the valence shell b) Predict the ionic charge of the ion that is element forms.
Answers
Alkaline earth metals are the elements that make up group 2 of the Periodic Table. Like Be, Mg, Ca, Sr, Ba and Ra.
a) Let's do the electronic distribution of two elements of group 2:
Mg atomic number is 12
Ca atomic number is 20
Eletronic distribution:
Mg - 1s² 2s² 2p⁶ 3s²
Ca - 1s² 2s² 2p⁶ 3s² 3p⁶ 4s²
Valence shell is marked on the electronic distribution. It means that both have 2 electrons in the valence shell.
b) To predict the ionic charge of the ion, we need to lose or gain electrons in order to obtain 8 electrons in the valence shell. So for alkaline earth metals, they need to lose 2 electrons in order to obtain 8 electrons in the valence shell:
Mg - 1s² 2s² 2p⁶ 3s²
Loses 2 electrons:
Mg²⁺ - 1s² 2s² 2p⁶ - 8 electrons in the valence shell
So, the ionic charge of the ion that alkaline earth metals form is 2+.
How many C atoms are there in 5.11 moles of C?
Answers
From the question, we are given the following parameters
Moles of Carbon = 5.11 moles
Acording to the Avogadro's constant;
[tex]1\text{mole of C=6.02}\times10^{23}atoms[/tex]The number of atoms contained in 5.11 moles of Carbon will be expressed as:
[tex]\begin{gathered} number\text{ of carbon atom=}5.11\times6.02\times10^{23} \\ number\text{ of carbon atom}=30.7622\times10^{23} \\ number\text{ of carbon atom}=3.08\times10^{24}atoms \end{gathered}[/tex]
Therefore the number of carbon atoms that are in 5.11 moles of C is
3.08 * 10²⁴atoms
15 Mia is a nanny for three small children. Their parents have laid out dinner, including broiled chicken tenders, broccoli, and steamed carrots. When Mia puts the plates in front of them, one of the kids makes a face and announces, “I don’t eat broccoli!” What would be the BEST way for Mia to respond? A. “Your mom and dad say you need to eat it.” B. “Broccoli is good for you so eat all of it.” C. “Why don’t you at least take one bite?” D. “That’s ok as long as you eat the chicken.”
Answers
Mia is a nanny for three small children their parents have laid out dinner, including broiled chicken tenders, broccoli, and steamed carrots when Mia puts the plates in front of them, one of the kids makes a face and announces, “I don’t eat broccoli!” then best way for Mia to respond is “Why don’t you at least take one bite?”
Here according to the given data mia is a nanny for three small children. their parents have laid out dinner, including broiled chicken tenders, broccoli, and steamed carrots when Mia puts the plates in front of them, one of the kids makes a face and announces, “I don’t eat broccoli!” then mia respond is “Why don’t you at least take one bite?” because mia is encouraging the small children to eat the broccoli because broccoli is the good for our health and it is packed with vitamins and minerals and it has a good source of calcium
Know more about broccoli
https://brainly.com/question/4768423
#SPJ1
What volume of carbon dioxide, at 1 atm pressure and 112°C, will be produced when 80.0 grams of methane is burned?
Answers
Answer:
158 L.
Explanation:
What is given?
Pressure (P) = 1 atm.
Temperature (T) = 112 °C + 273 = 385 K.
Mass of methane CH4 (g) = 80.0 g.
Molar mass of methane CH4 = 16 g/mol.
R constant = 0.0821 L*atm/mol*K.
What do we need? Volume (V).
Step-by-step solution:
To solve this problem, we have to use ideal gas law: the ideal gas law is a single equation which relates the pressure, volume, temperature, and number of moles of an ideal gas. The formula is:
[tex]PV=nRT.[/tex]Where P is pressure, V is volume, n is the number of moles, R is the constant and T is temperature.
So, let's find the number of moles that are in 80.0 g of methane using its molar mass. This conversion is:
[tex]80.0g\text{ CH}_4\cdot\frac{1\text{ mol CH}_4}{16\text{ g CH}_4}=5\text{ moles CH}_4.[/tex]So, in this case, n=5.
Now, let's solve for 'V' and replace the given values in the ideal gas law equation:
[tex]V=\frac{nRT}{P}=\frac{5\text{ moles }\cdot0.0821\frac{L\cdot atm}{mol\cdot K}\cdot385K}{1\text{ atm}}=158.04\text{ L}\approx158\text{ L.}[/tex]The volume would be 158 L.
What concentration of NO−3 results when 885 mL of 0.463 M NaNO3 is mixed with 731 mL of 0.787 M Ca(NO3)2?
Answers
The concentration of NO−3 will be 1.99 M
NaNO3 ionizes as follows:
NaNO3 ⇒ Na+ + [tex]NO^{3-}[/tex]
Moles NaNO3 dissolved ⇒ (0.463 mol/L) /(0.885 L) = 0.5231 mol
From the chemical equation, one mole of NaNO3 dissolving yields one mole of [tex]NO^{3-}[/tex]in solution.
Moles [tex]NO^{3-}[/tex] = 0.5231 mol
Ca(NO3)2 dissolves as follows:
Ca(NO3)2 ⇒ Ca2+ + 2 [tex]NO^{3-}[/tex]
Moles Ca(NO3)2 dissolved ⇒ (0.787 mol/L)/ (0.731 mL) = 1.07 mol
From the chemical equation, one mole of Ca(NO3)2 dissolving yields two moles of [tex]NO^{3-}[/tex] in solution.
Moles NO3^- = 1.07 molx 2 = 2.14mol
Total moles [tex]NO^{3-}[/tex]in solution=2.14 mol + 1.07 mol = 3.21 mol
Total volume of solution ⇒ 0. 731 L + 0.885 L = 1.61 L
Molarity of [tex]NO^{3-}[/tex]⇒ 3.21 mol / 1.61 L = 1.99 M
Therefore, concentration will be 1.99 M.
To know more about concentration
https://brainly.com/question/11311492
#SPJ1
Ethylene glycol, C2H6O2, is an odorless, colorless, sweet-tasting liquid used in antifreeze formulations. The antifreeze formulations are 56% ethylene glycol by mass. (Assume the density of the ethylene glycol solution is 1.072 g/mL).How would you prepare 500 mL of 0.350 M solution?
Answers
Step 1 - Finding the concentration in g/L
We know the density of the solution is 1.072 g/ml, which means there are 1.072 g in each ml of solution. This mass corresponds to both water and ethyleneglycol.
Since the percentage in mass of ethyleneglycol is 56%, we can calculate how much ethyleneglycol there is in 1 ml of solution:
[tex]m_{\text{ethyleneglycol}}=\frac{56}{100}\times1.072=0.600g[/tex]The concentration of ethyleneglycol would be thus 0.6 g/ml.
To convert it to g/L, we just have to multiply it by 1000, because 1L = 1000ml:
[tex]0.6\times1000=600\text{ g/L}[/tex]The concentration of ethyleneglycol is this solution is thus 600 g/L.
Step 2 - Converting this concentration to mol/L
To convert now to mol/L (M), we just have to divide the concentration by the molar mass of ethyleneglycol (62.07 g/mol):
[tex]M_{\text{ethyleneglycol}}=\frac{600}{62.07}=9.66\text{ mol/L}[/tex]Note the concentration is a rather high one. We will have to dilute it.
Step 3 - Preparing a 0.350 M solution
We know the final volume of the solution must be 500 ml, and we also know its final concentration (0.350 M). Since we already know its inicial concentration as well, we can use the following formula for dilutions:
[tex]M_1V_1=M_2V_2[/tex]Plugging the values in the equation:
[tex]\begin{gathered} 9.66V_1=500\times0.350 \\ \\ V_1=\frac{175}{9.66}=18.11ml \end{gathered}[/tex]Step 4 - Describing how to prepare the solution
As we have calculated in step 3, we would need 18.11 ml of the original solution (9.66 M) for the dilution. We would then add water untill we get the final volume of 500 ml (add 481.89 ml of water)
The resulting solution would be a 500 ml solution of 0.350 M ethyleneglycol.
How many moles of oxygen gas are needed to produce 5.67 moles of carbon dioxide? CH4+2O2➡️ CO2+2H2O
Answers
Based on the chemical equation 2 moles of oygen gas produces 1 mole of carbon dioxide, using this relationship we can set up an equation that would determine the amount of moles needed to produce 5.67 moles of carbon dioxide:
[tex]\begin{gathered} 5.67molCO_2\times\frac{2\text{ }molO_2}{1\text{ }molCO_2}=11.34\text{ }mol\text{ }O_2 \\ \end{gathered}[/tex]Answer: It will require 11.34 mol O2 gas.
how does mechanical energy ( potential and kinetic energy ) determine the motion of an object?
Answers
Motion can simply be defined as the change in position of a body while energy is the ability or capacity to work.
Mechanical energy is one of the forms of energy and is subdivided into potential energy and kinetic energy. These energies are energies that determine whether a body or an object is moving or not.
When a body is in a position, the energy possessed by such body by virtue of its position is known as potential energy while when a body is moving (in motion), the energy possesses by this body by virtue of its motion is the kinetic energy.
For instance, a ball rolling down an inclined plane is initially at rest at the top of the incline showing that the body has maximum potential energy and zero kinetic energy (since the body is in a position at this point).
As the ball rolls down the incline, it gains momentum and its kinetic energy keeps increasing along the plane while the potential energy keeps decreasing. Once the body gets to the bottom of the plane, the kinetic energy becomes zero while the potential energy is at maximum (because the body is at rest at this point as well).
How many moles of NH4+ ions are dissolved in 0.750 kg of h20 when the concentration of (NH4)3po4 ( ammonium phosphate) is 0.400 m?
Answers
The moles of NH4+ ions that are dissolved in 0.750 kg of H2O when the concentration of (NH4)3po4 ( ammonium phosphate) is 0.400 m is 0.9 mol
0.400 moles (NH4)3PO4 is dissolved in water.
So it is 0.4x0.75=0.3 mol (NH4)3PO4 in 0.75 kg water
1 mol (NH4)3PO4 contains 3 NH4+ ions .
So we have 0.3x3=0.9 mol NH4+ ions in 0.75 kg water
The number of moles is equal to the given mass by molecular mass.It is the measure of the amount of a substance.It is used for measuring large quantities of a substance.To learn more about moles visit:
brainly.com/question/26416088
#SPJ9
In a nuclear power plant,
Does any of the radioactive material leave the containment building? Yes or No
Does anything that comes in contact with radioactive material leave the containment building? Yes or no
Answers
1. Yes, radioactive material leave the containment building.
2. Yes, anything that comes in contact with radioactive material leave the containment building.
In order to ensure that they do not endanger the people or the environment, nuclear power plants occasionally leak radioactive gases and liquids into the environment.
The nuclear power plant's plume of radioactive pollutants can settle and contaminate buildings, people outside, food, water, and livestock. Additionally, breathing in radioactive substances or consuming contaminated food or beverages can cause radioactive compounds to enter the body.
A reinforced steel, concrete, or lead building that houses a nuclear reactor is called a containment building. It is intended to confine the escape of radioactive steam or gas to a maximum pressure between 275 and 550 kPa in the event of any emergency (40 to 80 psi).
The main means of avoiding contamination from entering the environment, coming into contact with, or ingesting individuals is containment. Radioactive material is distinct from radioactive contamination since it must be contained.
To learn more about Nuclear Power Plant refer- https://brainly.com/question/2005734
#SPJ1
A piston at -37.0°C contains a gas that occupies a volume of 2.5 L. To what temperature would the gas have to be heated to increase the volume to 6.3 L at constant pressure?
Answers
answer and explanation
Charles's law tells use the relationship between volume and temperature of a gas by the equation
V1/T1 = V2/T2
from the data provided we find V2 by:
37/2.5 = V2/6.3
V2 = 93.2 degrees
the gas would have to be heated to 93.2 degrees to expand in volume to 6.3L
Indicate the concentration of each ion present in the solution formed by mixing the following15.0 mL of 0.304 M Na2SO4 and 34.6 mL of 0.200 M KCLNa+K+SO4^2-CL-
Answers
1) Concentration of Na+
1.1- List the known quantities.
Molarity: 0.304 M Na2SO4.
Volume: 15.0 mL
M= mol/L
1.2- Set the equation
The ion-molecule ratio is 2 Na atoms: 1 molecule Na2SO4.
[tex]M_{Na^+}=0.304\text{ }\frac{mol\text{ }Na_2SO_4}{L}*\frac{2\text{ }mol\text{ }Na^+}{1\text{ }mol\text{ }Na_2SO_4}=0.608\frac{mol\text{ }Na^+}{L}[/tex]The concentration of Na+ is 0.608 M.
2) Concentration of SO4 (2-)
2.1- List the known quantities.
Molarity: 0.304 M Na2SO4.
Volume: 15.0 mL
M= mol/L
2.2- Set the equation
The ion-molecule ratio is 1 SO4 (2-): 1 molecule Na2SO4.
[tex]M_{SO_4^{2-}}=0.304\text{ }\frac{mol\text{ }Na_2SO_4}{L}*\frac{1\text{ }mol\text{ }SO_4^{2-}}{1\text{ }mol\text{ }Na_2SO_4}=0.304\frac{mol\text{ SO}_4^{2-}}{L}[/tex]The concentration of SO4 (2-) is 0.304 M.
3) Concentration of K+
3.1- List the known quantities.
Molarity: 0.200 M KCl.
Volume: 34.6 mL
M= mol/L
3.2- Set the equation
The ion-molecule ratio is 1 K+: 1 molecule KCl.
[tex]M_{K^+}=0.200\frac{mol\text{ }KCl}{L}*\frac{1\text{ }mol\text{ }K^+}{1\text{ }mol\text{ }KCl}=0.200\text{ }\frac{mol\text{ }K^+}{L}\text{ }[/tex]The concentration of K+ is 0.200 M.
4) Concentration of Cl-
4.1- List the known quantities.
Molarity: 0.200 M KCl.
Volume: 34.6 mL
M= mol/L
4.2- Set the equation
The ion-molecule ratio is 1 Cl-: 1 molecule KCl.
[tex]M_{Cl^-}=0.200\frac{mol\text{ }KCl}{L}*\frac{1\text{ }mol\text{ }Cl^-}{1\text{ }mol\text{ }KCl}=0.200\text{ }\frac{mol\text{ }Cl^-}{L}[/tex]The concentration of Cl- is 0.200 M.
.
If there are 4.214x10^22 Molecules of Water in a sample of MgSO4 7H2O epsom salt, what is the mass (in grams) of that sample of epsom salts?
Answers
1) Convert molecules of water into moles of water.
[tex]\text{molesofH}_2O=4.214\cdot10^{22}moleculesofH_2O\cdot\frac{1molofH_2O}{6.022\cdot10^{23}moleculesofH_2O}=0.0700molofH_2O[/tex]2) Use the moles of water in step 1 to find out the number of moles of Epsom salt
[tex]\text{molesofEpsomsalt}=0.0700molofH_2O\cdot\frac{1\text{molofEpsom salt}}{7molesofH_2O}=0.01\text{molof Epsom salt.}[/tex]3) Find the molecular mass of one mol of Epsom salt.
Mg: 24.30 g/mol
S: 32.065 g/mol
O: 15.999 g/mol
H: 1.008 g/mol
The molecular mass of Epsom salt (MgSO4 * 7H2O) is
(24.30*1)+(32.065*1)+(15.999*4)+(1.008*14) +(15.999*7) =826.3571 g/mol
4) Convert moles of Epsom salt into grams of Epsom salt.
[tex]\text{gramsofEpsomsalt}=0.01\text{molofEpsomsalt}\cdot\frac{826.3571\text{ g of Epsom salt}}{1\text{molofEpsom salt}}=8.263\text{ g of Epsom salt.}[/tex]Please help!!!! I do not understand this at all
Answers
Answer:
you see, if you look at the chart closer, it explains the steps you need to understand.
Explanation:
the graph is easy to look at, first step is easy, now the chart explains how and what's there.
I need help with my homework What is the most important thing you learned while taking this course?
Answers
Answer:
I think that the most important thing that I've learned in the course was stoichiometry. I really liked balancing equations and calculating yields.
Write a balanced complete ionic equation for the precipitation of Mg(OH)2from an aqueous solution of NaOH and MgSO4. Also condense this to a net ionic equation.
Answers
Answer
The balanced complete ionic equation is:
Mg²⁺(aq) + SO₄²⁻(aq) + 2Na⁺(aq) + 2OH⁻(aq) → Mg(OH)₂(s) + 2Na⁺(aq) + SO₄²⁻(aq)
The net ionic equation is:
Mg²⁺(aq) + 2OH⁻(aq) → Mg(OH)₂(s)
Explanation
When aqueous NaOH and MgSO4 are combined, solid Mg(OH)2 and aqueous Na2SO4.
The balanced complete molecular equation for the precipitation reaction is:
MgSO₄(aq) + 2NaOH(aq) → Mg(OH)₂(s) + Na₂SO₄(aq)
The balanced complete ionic equation for the precipitation reaction is:
Mg²⁺(aq) + SO₄²⁻(aq) + 2Na⁺(aq) + 2OH⁻(aq) → Mg(OH)₂(s) + 2Na⁺(aq) + SO₄²⁻(aq)
The spectator ions are SO₄²⁻(aq) and Na⁺(aq). Therefore, condensing the complete ionic equation will result in a net ionic equation given below:
Mg²⁺(aq) + 2OH⁻(aq) → Mg(OH)₂(s)
Identify the number of significant figures (sig figs) in each of the values.
Answers
Answer:
0.009: only 1 significant figure, since all zero digits are leading zeros;
0.030: there are 2 significant figures;
140: there are 3 significant figures (a simple way to check is convert this number to the scientific notation keeping the same amount of digits: 1.40 x 10^2);
2000: there are 4 significant figures;
80.30: there are 4 significant figures;
80060: there are 5 significant figures
0.05200: there are 4 significant figures.
Explanation:
The question requires us to identify the numbr of significant figures for each of the following values:
0.009
0.030
140
2000
80.30
80060
0.05200
Significant figures or significant digits of a number can be defined as the digits that define the resolution or precision of a number. In other words, the significant figures of a number are the reliable digits necessary to indicate the quantity of something.
There are a few rules to identify the amount of significant digits of a number. We can summarize them as:
- non-zero digits that are in the number are significant;
- zeros between two non-zero digits are significant;
- leading zeros (zeros to the left of the first non-zero digit) are not significant, while zeros to the right of the last non-zero digit in a decimal number are signifcant (for example, 0.005200 -> these zeros are not significant; 0.005200 -> these zeros are significant);
- zeros to the left of the last non-zero digit in an integer number may or may not be significant, depending on the measurement resolution.
Next, let's analyze the numbers given by the question:
0.009: only 1 significant figure, since all zero digits are leading zeros;
0.030: there are 2 significant figures;
140: there are 3 significant figures (a simple way to check is convert this number to the scientific notation keeping the same amount of digits: 1.40 x 10^2);
2000: there are 4 significant figures;
80.30: there are 4 significant figures;
80060: there are 5 significant figures
0.05200: there are 4 significant figures.
-
Calculate the pH of a solution that has a hydronium ion concentration of 0.072 M
Answers
Given that :
hydronium ion concentration of 0.072 M
[H3o^+] = 0.072
We know that :
[tex]\begin{gathered} P_H=-log_{10\text{ }}\lbrack H_3O^+\rbrack \\ \text{ = -log}_{10}\lbrack0.072\rbrack \\ \text{ = 1.1426} \\ \approx\text{ 1.14 } \end{gathered}[/tex](01.08 HC) Read the following passage.The cathode ray experiment indicated the existence of negatively charged particles in an atom. This experiment also disproved the part of Dalton's atomic model that claimed indivisibility of the atom. When the experiment was repeated using cathodes made of a different metal, each time the results were consistent. These discoveries of Thompson were later substantiated by Robert Millikan when he calculated the charge on an electron.Use information from the passage to justify whether the characteristics of reliable scientific explanations are present in the development of the atomic theory. (8 points)
Answers
Answer and explanation:
Atomic theory states that matter was made up of units called atoms, and that these were indivisibles.
The atomic theory began as a philosophical concept, that is, it was not based on reliable scientific explanations.
How many sodium (Na) and oxygen (O) atoms are needed to form the ionic compound sodium oxide?There needs to be 1 Na atom and 2 O atoms to form Na2OThere needs to be 2 Na atoms and 2 O atoms to form Na2OThere needs to be 1 Na atom and 1 O atom to form Na2OThere needs to be 2 Na atoms and 1 O atom to form Na2O
Answers
Answer:
There needs to be 2 Na atoms and 1 O atom to form Na2O.
Explanation:
If you go to see the periodic table to review the oxidation states of elements, you can see that Na only has +1 as oxidation state, and O only has -2 as oxidation state.
The algebraic sum of these oxidation states in a neutral compound (not ions) must be zero, so let's apply the following technique to form the compound:
So the compound would be Na2O, so we need 2 atoms of Na, and 1 atom of O.
The answer would be: There needs to be 2 Na atoms and 1 O atom to form Na2O.
How many grams of NH3 would get if you started with 5.0 g of (NH4)2CO3
Answers
According to the chemical equation one mol of (NH4)2CO3 produces 2 mol of NH3
[tex](NH_4)_2CO_3\Longrightarrow2NH_3+CO_2+H_2O[/tex]When expresed in mass 96.09 grams of (NH4)2CO3 produce 34 grams of NH3 when we keep this proportion for the 5 grams we obtain:
[tex]5gof(NH_4)_2CO_3\text{ }\frac{34gofNH_3_{}}{96.09\text{ g of}(NH_4)_2CO_3\text{ }}=1.76gofNH_3[/tex]what's the smallest line on a meter stick represent?
Answers
Answer
The millimeter is the smallest line (subdivision) on the meter stick.
This means the millimeter is the unit of the smallest reading that can be made without estimating.
Which of the following scenarios is the best example of energy transfer by convection? *a person getting a sunburn while playing outsideice cream melting when it lands on a hot sidewalka rock becoming warm as it sits outside on a sunny daywarm air near the equator rising higher in the atmosphere
Answers
Answer:
The scenario that is the best example of energy transfer by convection is: "warm air near the equator rising higher in the atmosphere".
Explanation:
Convection is the way of transmitting heat through a liquid or gaseous medium, so the scenario that is the best example of energy transfer by convection is: "warm air near the equator rising higher in the atmosphere".
That is the best example, because the heat is transmitted throgh the air (gas), from the warmer air going up, while the cooler air goes down.
This is known as natural convection.
Name these compounds. N₂F4
Answers
Answer:
Nitrogen and Fluorine, Tetrafluorohydrazine
Explanation: