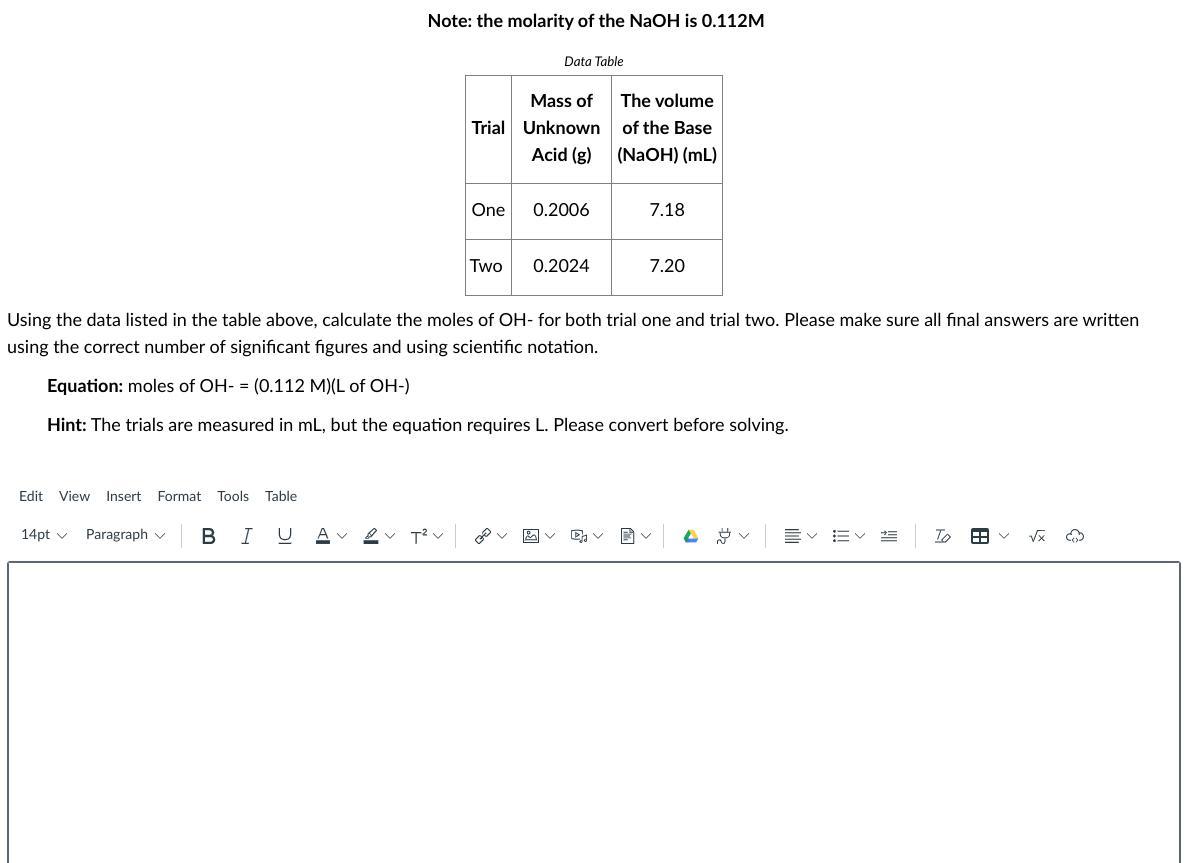
Answers
Explanation:
To solve this question, we need to use the following equation:
M = n/V
where:
M = molarity
n = number in moles
V = volume
For trial 1 we have:
M of NaOH = 0.112 mol/L
n = ??
V = 7.18 mL (we need to transform it to L, just divide by 1000)
V = 0.718 L
So:
M = n/V
n = M*V
n = 0.112*0.718
n = 0.080 moles of NaOH
The equation of dissociation of NaOH is:
NaOH --> Na+ + OH-
0.080 0.080 0.080
Answer for trial one: 0.080 moles of OH- or 8.0x10^-2 moles
For trial 2 we have:
For trial 1 we have:
M of NaOH = 0.112 mol/L
n = ??
V = 7.20 mL (we need to transform it to L, just divide by 1000)
V = 0.720 L
So:
M = n/V
n = M*V
n = 0.112*0.720
n = 0.081 moles of NaOH
The equation of dissociation of NaOH is:
NaOH --> Na+ + OH-
0.081 0.081 0.081
Answer for trial two: 0.081 moles of OH- or 8.1x10^-2 moles
Related Questions
Why does an increased temperature cause a reaction to occur faster?A. It does not. The increased temperature causes the particles to spread out and collide less.B. The increased temperature makes the molecules more loosely attached so they can switch atoms more easily.C. The increased kinetic energy causes the particles to move faster, causing more collisions.D. The increased potential energy in the particles means less energy is needed from the environment for the activation energy.
Answers
Answer
C. The increased kinetic energy causes the particles to move faster, causing more collisions.
Explanation
An increased temperature typically causes a reaction to occur faster by raising the average kinetic energy of the reactant molecules.
Therefore, a greater proportion of molecules will have the minimum energy necessary for an effective collision.
The correct answer is option C. The increased kinetic energy causes the particles to move faster, causing more collisions.
2NaOH + H2504 ® Na2504 + 2H20What is the mole ratio of sodium hydroxide to water?
Answers
Given the balanced equation :
• 2,NaOH + H2S04 → Na2S04 + ,2,H20
2 : 1 :1 :2
We can see that 2 moles of NaOH produces 2 moles of H20
The ratio is 2: 2 which is the same as 1: 1 , because for every mole of NaOH , an equal amount of moles for water will be produced.The phase diagram of a substance is given below. This substance is a __ at 10 °C and 1 atm.
Answers
The phase diagram of a substance is given below. This substance is a solid at 10 °C and 1 atm.
When a material switches from one state of matter to another, a phase transition takes place. Matter exists in three basic states: liquid, solid, and gas. The many phases of a material are shown on a phase diagram using numerous variables, most frequently temperature and pressure. The figure may be used to illustrate how altering these factors impacts a substance's state of matter.
The fusion (melting or freezing) curve, which depicts the change from a liquid to a solid state. The curve that depicts the change from a gaseous to a liquid state is called the vaporization (or condensation) curve. The sublimation (or deposition) curve depicts the change from a gaseous to a solid state.
To know more about phase diagram visit : brainly.com/question/14718830
#SPJ1
if a compounds of calcium oxide has a mass of 5.45 grams what would be the number of moles for this mass
Answers
To answer this question, we have to use calcium oxide molar mass. It is 56.1g/mol, which means that 1 mol of this compound is 56.1 grams. Use this to find how many moles are there in 5.45grams of calcium oxide:
[tex]5.45gCaO\cdot\frac{1molCaO}{56.1gCaO}=0.097molCaO[/tex]It means that for this mass, there are 0.097 moles of CaO.
If the pressure of a gas sample is increases 4x and the absolute temperature remains the same, by what factor does the volume of the sample change?A. 1/4B. 02C. 1/2D. 08
Answers
Answer:
The factor of change is 1/4.
Explanation:
Since in this case we have a constant temperature, the changes in the pressure will affect the volume like the Boyle's Law formula explains:
[tex]\begin{gathered} P_1*V_1=P_2*V_2 \\ \end{gathered}[/tex]To find the change of the sample volume, let's replace the initial pressure (P1) with A, and let's increase the pressure (P2) 4x:
[tex]\begin{gathered} P_1*V_1=P_2*V_2 \\ A*V_1=(A*4)*V_2 \\ \frac{A*V_1}{A*4}=V_2 \\ \frac{1}{4}V_1=V_2 \end{gathered}[/tex]So, the factor of change is 1/4.
Balance the following equation using the change in oxidation numbered method. Ag +H2 S +O2 —> Ag2 S + H2O
Answers
To balance an equation with the oxidation numbered method we must write the oxidation states of the elements that participate in the reaction.
[tex]Ag_^0+H_2^{+1}S^{-2}+O_2^0\rightarrow Ag_2^{+1}S^{-2}+H_2^{+1}O^{-2}[/tex]Once we have the oxidation states we must identify how they change from reactants to products. Thus we identify which are oxidized and which are reduced. If its oxidation state increases it is because the element is oxidized, if it decreases it will have been reduced.
We see that the elements that change their oxidation state are silver, Ag and Oxygen. We have that:
13. Choose the geologic formation that fits the description: the world's longest mountain chain.
Answers
The mid-ocean ridge would be the longest and largest mountainous region. With a circumference of 40,389 miles, it genuinely is a world landmark.
The water covers around 90% of the mid-ocean ridge system. The world is crisscrossed by a system of mountains as well as valleys that resembles the seams in a baseball. Tectonic plate movement on Earth is what creates it.
Mountain ranges like the Rockies had also often grown and fallen over the evolution of our planet. Whenever two continents clash, mountains are created. Due to their identical weight as well as diameter, neither plate would bury beneath the other.
In a process known as plate tectonics, sections of such Earth's mantle, known as plates, collide with one another as well as buckle up like such a rear bumper in a frontal collision to build the world's largest mountain ranges.
To know more about mountain chain
https://brainly.com/question/29276761
#SPJ1
Which of the following are examples of physical changes? Select all that applySteel rustingPaper burningAcetone evaporatingIce melting
Answers
Physical changes are changes in which there are no changes in the internal structure of substances. When there is a chemical change a chemical reaction is involved and the internal structure of the substance changes.
Of the four options that give us the first two: The oxidation of steel and burned paper represents an oxidation and combustion reaction respectively, so they correspond to chemical changes.
The second two options are phase changes, there is no chemical reaction and therefore the internal structure of the substances does not change. So the options to select will be:
Answer:
Acetone evaporating
Ice melting
An atom of which of the following elements has the greatest ability to attract electrons? Select one: a. sulfur b. nitrogen c. silicon d. chlorine
Answers
Explanation:
Electronegativity corresponds to the ability of the nucleus of an atom to attract the electrons involved in a chemical bond.
Elements belonging to the same period (same row) of the Periodic Table, we will see that electronegativity increases from left to right.
Elements belonging to the same group (same column), electronegativity increases from bottom to top.
The element among sulfur, nitrogen, silicon and chlorine that has the higher eletronegativity is chlorine.
Answer: d. chlorine
Which of the following choices is NOT an example of a colligative property?A. Vapor pressure loweringB. Freezing point depressionC. Boiling point elevationD. Melting point acceleration
Answers
Explanation: Collagative properties are characteristics of a solution that is dependent on the ratio of solute particles to to solvent particles.
Answer: D) Melting point acceleration.
Mass (M) will measured in which unit?O grams (g)O kelvin (k)O milliliters(ml)O Seconds (s)
Answers
According to these options presented here, we can see that:
Kelvin is for temperature
Milliliters are for volume
Seconds is for time
So, the answer for mass is grams (g). Therefore, we measure the mass in grams.
Answer: grams (g)
How to identify Bo . compounds
Answers
Identify Bo compounds as the quantitatively by the green coloration
The presence of boron compound can be detected quantitatively by green coloration they impart to the flame of an ordinary laboratory or bunsen burner and also boron can be identified by using spectrophotometric and colorimetric method and the chemical properties of boron are more similar to carbon and silicon than element of its own group although boron is the more electron dificient
Know more about boron
https://brainly.com/question/11651796
#SPJ1
Select the correct answer from each drop-down menu. How can you reduce air pollution arising from transportation?
Answers
Explanation:
When using public transport vehicles instead of private cars, we emit less pollution. For example in a bus more than 20 people travel, so the pollution per person is lower.
When we increase speed we usually consume more fuel, so avoiding accelerating rapidly we also reduce pollution.
Answer:
a) public transport vehicles.
b) accelerating rapidly.
Determine the pressure change when a constant volume of gas at 1.00 atm is heated from 20.0°c to 30.0° c show your work how to solve
Answers
In order to solve this question, we will be using the Gay-Lussac's gas Law, which is an experimental gas law that shows the relationship between temperature and pressure for a gas, the formula is:
P1/T1 = P2/T2
We have:
P1 = 1.00 atm
T1 = 20.0°C, but we need it Kelvin, therefore, 293 K
P2 = ?
T2 = 30°C, or 303 K
Now we add these values into the formula:
1/293 = P2/303
P2 = 1.03 atm is the new pressure
convert 12 liters to barrels
Answers
Answer:
12 liters is equal to 0.075 barrels.
Explanation:
To convert liters into barrels, it is necessary to use the following conversions:
- 1 barrel = 42 gallons
- 1 liter = 0.264 gallons
We have to write each conversion as a quotient:
[tex]12L*\frac{0.264gallons}{1L}*\frac{1barrel}{42gallons}=0.075barrels[/tex]We must write each conversion in a way that the liters cancel out, then the gallons cancel out in order to obtain the result in barrels.
So, 12 liters is equal to 0.075 barrels.
What conditions are needed for large storm formation?
Answers
Answer:
Lifting, instability, and moist/humid air
Explanation:
The water is released from the air, forming storm clouds, while humid air is flowing upward at a zone of low pressure over warm ocean water. The air in a hurricane rotates as it rises.
After swimming in the pool on a very hot day, Sarah had a glass of ice water. Over time, the water on her skin evaporated, and the ice cubes melted in the glass. Howmany states of water existed in this scenario?A. 3B. 1C. 4D. 2
Answers
3 states
Explanations:Matter can exist in three forms namely;
• Solid state
,• Liquid state
,• Gaseous state
From the given scenario, water is known to exist as ice cubes (which is the solid state). Also since the water Aon her skin evaporated the water changed to steam (gaseous state) in this case.
Also note that the ice cubes melted in the glass. This melted ice changes to liquid ,at this point. Therefore, we can conclude that the water in the scenario exists in three forms (Liquid, Solid and Gas).
A physician orders a Heparin drip at 8.0 units per kg of body weight per hour via IV pump. The patient weighs 237lb. The IV is available at 25,000 units of Heparin in exactly 500 mL of IV fluid. Calculate the flow rate in mL/hr that should be set for the IV pump.
Answers
The flow rate of the IV pump should be 17.06mL/hr.
1st) We need to calculate the patient's weight knowing that 1 lb is equal to 0.45 kg:
[tex]\begin{gathered} 1\text{ lb-0.45 kg} \\ 237\text{ lb - x =}\frac{237\text{ lb}\cdot\text{0.45 kg}}{1\text{ lb}} \\ \\ x=106.65\operatorname{kg} \end{gathered}[/tex]The patient weighs 106.65 kg.
2nd) If the patient needs a drip at 8.0 units per kg of body weight, we have to calculate the units for the patient:
[tex]\begin{gathered} 1\text{ kg - 8.0 units} \\ 106.65\text{ kg - x=}\frac{106.65\text{ kg}\cdot\text{8.0 units}}{1\text{ kg }} \\ \\ x=853.2\text{units} \end{gathered}[/tex]So, the patient needs 853.2 units per hour.
3rd) To calculate the flow rate of the IV pump, we need to know the mL equivalent to 853.2 units:
[tex]\begin{gathered} 25,000\text{ units - 500mL} \\ 853.2\text{ units - x=}\frac{853.2\text{ units}\cdot\text{500mL}}{25,000\text{ units}} \\ \\ x=17.06mL \end{gathered}[/tex]So, the flow rate of the IV pump should be 17.06mL/hr.
The question is in the image. If you think the question has an incorrect answer selected, then select the correct answer. Furthermore, if you can try to explain why the answer is correct and/or incorrect, I will also leave attached a text that may help you answer the question.
Answers
Compared to the normal freezing and boiling point of water, adding sugar to water will result in:
D. Lower freezing point and higher boiling point.
The chosen answer is the correct one.
This is explained as follows:
When we add sugar to water we form a solution, water molecules sorroud sugar molecules interacting with them electrostatically, this results in less interaction between water molecules and a lower amount of energy needed for them to maintain their freedom, so they will stay in liquid state at lower temperatures.
On the other hand, when we take the solution to its boiling point we will see that it is higher than that of pure water. This happens because the interaction between water and sugar molecules makes it harder for water molecules to escape the liquid phase, increasing the boiling temperature.
Use the equation below to answer (What do you need to do first???)C3H8, + 02 →CO2,+ H2O1. How many moles of CO2, are produced for each mole of C3H8 used?2. If you start with 362 grams of C3H8 how many grams of H2O will be produced?
Answers
a) To get the number of moles of CO2 that are produced for each mole of C3H8 used, we will use the stochiometry ratio from the balanced chemical reaction.
The balanced chemical reaction that resulted from the combustion of propane gas is given as:
[tex]C_3H_8+5O2\to3CO_2+4H_2O[/tex]From the balanced reaction, you can see based on stoichiometry that for each mole of propane that reacted, 3 moles of CO2 was produced.
b) If you start with 362 grams of C3H8, the number of moles of propane will be expressed according to the formula:
[tex]\text{moles of C}_3H_8=\frac{Mass}{Mola\text{r mass}}\text{ }[/tex]Given the following parameters:
Mass of propane gas = 362 grams
Molar mass of propane = (3*12) + (1*8) = 36 + 8 = 44g/mol
[tex]\begin{gathered} \text{Moles of C}_3H_8=\frac{362}{44} \\ \text{Moles of C}_3H_8=8.227moles \end{gathered}[/tex]From stoichiometry, you can see that 1 mole of C3H8 produces 4 moles of H2O, hence the moles of H2O that will be produced is given as:
[tex]\begin{gathered} \text{Moles of H}_2O=4\times8.227 \\ \text{Moles of H}_2O\approx32.91\text{ moles} \end{gathered}[/tex]Get the mass of H2O that was produced using the formula:
[tex]\begin{gathered} \text{Mass of H}_2O=moles\text{ }\times molar\text{ mass} \\ \text{Mass of H}_2O=32.91\cancel{\text{moles}}\times\frac{18g}{\cancel{\text{mol}}} \\ \text{Mass of H}_2O=32.91\times18g \\ \text{Mass of H}_2O=592.38\text{grams} \end{gathered}[/tex]Therefore if you start with 362 grams of C3H8, 592.38grams of H2O will be produced
An aqueous solution (water is the solvent) of a KCl compound ( l = 2 ) boils at 102.5 degrees celcius. What is the molality (m) of the solution? Assume that the pure water boils at 100 degrees celcius.
Answers
Explanation
Given:
i = 2
boiling point of water = 100 'C
KCl boiling point = 102.5 'C
Required: Molality (m) of the solution
Solution
DT = i x Kb x m
DT = 102.5-100 = 2.5 'C
DT = i x Kb x m
2.5 = 2 x 0.512 x m
2.5 = 1.024m
molality = 2.44 m
Answer
Molality = 2.44 m
What polyatomic ions have a 3- charge?Check all that apply
Answers
Answer:
Phosphate ion only
Explanation:
Here, we want to get the polyatomic ions with a charge of -3
What we have to do here is to write the polyatomic ions and their formula
We have this as follows:
[tex]\begin{gathered} \text{Phosphate ion - PO}^{3-}_4 \\ \text{Acetate ion - }C_2H_3O^-_2 \\ Sulfate-SO^{2-}_{4^{}} \\ Hydro\times ide-OH^- \\ \\ \text{Dichromate - Cr}_2O^{2-}_7 \\ \text{Hydrogen phosphate - HPO}^{2-}_4 \end{gathered}[/tex]From the above, the polyatomic ions with a charge of -3 is the Phosphate ion only
A 0.333 g-sample of antacid was dissolved in 40.00 mL of 0.135 MHCI solution, then back-titrated to the end-point with 9.28 mL of a0.0203 M NaOH solution.a) Calculate number of moles of acid in the original 40.00 mL of HCI?b) How many moles of base were used in the back titation of excessHCI?c) How many moles of excess HCII?a How many moles of HCl reacted with the antacid sample?
Answers
A) To find the number of moles of a compound, we can use the Molarity formula, which tells us that molarity is equal to the number of moles divided by the volume in liters.
M = n/V
Now in our question we have a few informations, the molarity and the volume
0.135M = n/0.04L
Therefore the number of moles will be:
n = 0.135M * 0.04L
n = 0.0054 moles or 5.4 * 10^-3 moles
B) Using the same molarity equation as it was used in letter A, we can find the number of moles:
M = n/V
0.0203M = n/0.00928
n = 0.000188 moles or 1.88 * 10^-4
C) Since the ratio of this reaction HCl + NaOH is 1:1, which means I need 1 mol of HCl to react with 1 mol of NaOH, we can easily find the excess by subtracting both numbers of moles:
Moles of HCl - Moles of HCl that reacted
0.0054 - 0.00018 = 0.0052 of excess of HCl or 5.2 * 10^-3
HCl + NaOH -> NaCl + H2O
For the reaction NO(g) + 1/2 O2(g) -----> at 750 degrees Celsius, the equilibrium constant Kc equals
Answers
Explanation:
Given the following reaction.
NO (g) + 1/2 O₂ (g) <---> NO₂ (g)
We have to find the relationship between its Kc and Kp.
The Kp can be calculated from the Kc using the following expression.
Kp = Kc * (R*T)^(Δn)
Where Δn is the sum of the coefficients of the products minus the sum of the coefficients of the reactants. So Δn will be equal to:
Δn = Σ n of products - Σ n of reactants
Δn = 1 - (1 + 1/2)
Δn = -1/2
So we can say that:
Kp = Kc * (R*T)^(Δn)
Kp = Kc * (R*T)^(-1/2)
Kc = Kp * (R*T)^(1/2)
Answer: Kc = Kp * (R*T)^(1/2)
How many bonds are broken when performing the reaction? How many bonds are formed?(Reminder to check if the reaction is balanced)
Answers
Explanation:
We have to find the bonds that are broken and formed during the following reaction.
__ C₂H₄ + __ O₂ ---> __ CO₂ + __ H₂O
First we have to balance the reaction. To balance a combustion reaction we usually start balancing the atoms of C. We have two on the left and 1 on the right side of the equation. So the coefficient for CO₂ must be 2.
__ C₂H₄ + __ O₂ ---> 2 CO₂ + __ H₂O
Then we balance the H atoms by changing the coefficient for water. We have 4 atoms of H on the left and two on the right. The coefficient must be 2.
__ C₂H₄ + __ O₂ ---> 2 CO₂ + 2 H₂O
And finally we can balance the O atoms by changing the coefficient for O₂. We have 6 O atoms on the right side, so the coefficient for O₂ must be 3. The balanced equation is:
C₂H₄ + 3 O₂ ---> 2 CO₂ + 2 H₂O
Now to determine the bonds that have to be broken we have to pay attention to the Lewis structures of the reactants. They are:
We have to break 4 C-H bond, 1 C=C and 3 O=O bond (since the coefficient for oxygen gas is 3).
The bonds that we have to form are:
We have to form 4 C=O bonds (since the coefficient for carbon dioxide is 2), and 4 O-H bonds (since the coefficient for water gas is 2).
Answer: Four C-H, one C=C and three O=O bond need to break up. Four C=O and 4 O-H bonds need to form. (second option)
I need to solve it and fill in the spaces
Answers
In this stoichiometry question, we have the following reaction:
4 Al + 3 N2 -> 2 Al2N3
We have:
2.5 grams of N2
We want to know how much Al we need in order to react with 2.5 grams of N2, and in order to do that, we need to find out how many moles of N2 we have in 2.5 grams, we will be using its molar mass, 28g/mol to do it:
28g = 1 mol
2.5g = x moles
28x = 2.5
x = 2.5/28
x = 0.089 moles of N2 in 2.5 grams
Now according to the molar ratio, we have 4 moles of Al for every 3 moles of N2, therefore, if we have 0.089 moles of N2:
4 Al = 3 N2
x Al = 0.089 N2
x = 0.119 moles of Al
Now we can find the mass needed, using the number of moles, 0.119 moles, and also the molar mass, 27g/mol:
27g = 1 mol
x grams = 0.119 moles of Al
x = 3.2 grams of Al is required
Answer is 3.2 grams
In a storage area of a hospital where the temperature has reached 55 °C, the pressure of oxygen gas in a 15.0-L steel cylinder is 965 Torr.What is the final pressure, in millimeters of mercury, when the temperature of the oxygen gas drops to 24 °C, and the volume and the amount of the gas do not change?
Answers
Answer:
[tex]873.80\text{ mmHg}[/tex]Explanation:
Here, we want to get the final pressure
From the pressure law, volume and temperature are directly proportinal
Mathematically:
[tex]\frac{P_1}{T_1}\text{ = }\frac{P_2}{T_2}[/tex]Where:
P1 is the initial pressure which is 965 torr
P2 is ?
T1 is the initial temperature which we convert to Kelvin by adding 273 K(55 + 273 = 328 k
T2 is the final temperature which is 24 + 273 = 297 K
Substituting the values, we have it that:
[tex]\begin{gathered} \frac{965}{328}\text{ = }\frac{P_2}{297} \\ \\ P_2\text{ = }\frac{297\times965}{328}\text{ = 873.80 torr} \end{gathered}[/tex]Now, we convert this to mmHg
Mathematically, 1 torr = 1 mmHg
We have the final pressure as 873.80 mmHg
What is the term for a hydrate with the water turned off?Answer choices:- waterless- anhydrous- dried- dehydrated
Answers
We can change a hydrate into anhydrous compound by heating it to drive off the water (dehydrate )
Correct choice is therefore anhydrous . Option 2 .
If you have 4.2x10^28 Zn (zinc) atoms, how many moles of zinc do you have?
Answers
We can find the number of moles of a compound based on their number of atoms using Avogadro's number which says that there are 6.022 x 10^(23) atoms in 1 mol.
[tex]4.2\cdot10^{28}atoms\text{ Zn}\cdot\frac{1\text{ mol Zn}}{6.022\cdot10^{23}atoms\text{ Zn}}=69744.27\text{ moles Zn.}[/tex]The answer is that there are 69744.27 moles of zinc in 4.2 x 10^(28) atoms of zinc.
I need assistance on number 5 including all parts of number 5 thank you !
Answers
5a) Propyne is a member of the alkyne group with the formula C3H4. The compound consists of 3 carbon atoms and 4 hydrogen atoms. The expanded form of the compound is as shown below;
b) For the skeletal structure of 2, 2 - dimethyl butane, the carbon and hydrogen atoms are hidden. The structure will only be written in form of lines as shown:
c) The molecular formula for benzene is C6H6. It is cyclic in nature with a total of 6 carbon atoms and 6 hydrogen atoms. The structure of the atom is as shown below;
d) Heptane is an alkane compound with the molecular formula C7H16. The condensed formula do not contain any lines or angles. They are written in term of their hydrogen and carbon compounds.
[tex]CH_3CH_2CH_2CH_2CH_2CH_2CH_3[/tex]