Answers
Answer:
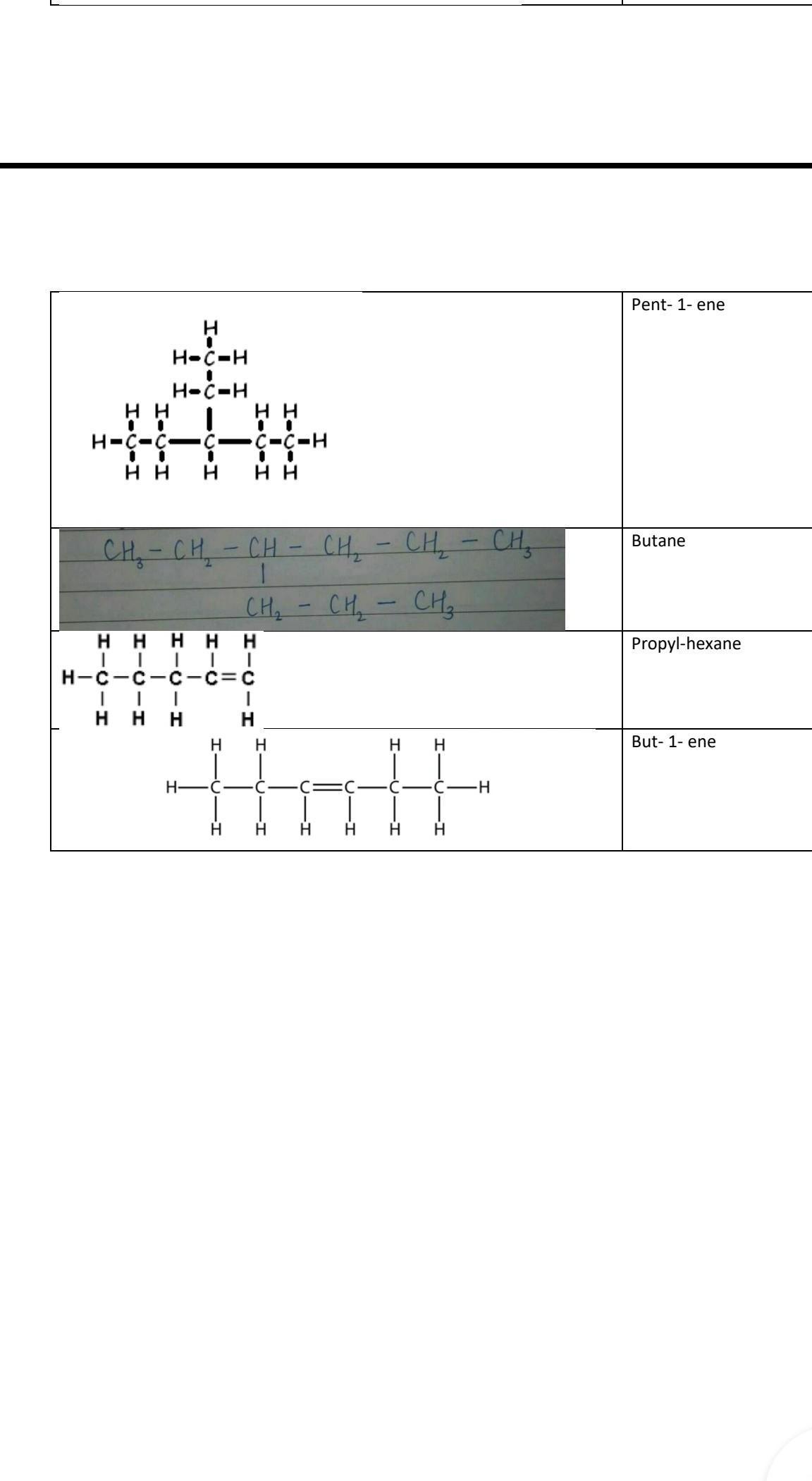
3_ethylpentane
4_ethylheptane
1_pentene
3_hexene
Related Questions
what is the noble gas configuration of V+3
Answers
Answer:
Ar 3d3 4s2
Explanation:
A 2.00 g sample of ammonia (NH3) reacts with 4.00 g of oxygen (O2) according to the equation 4NH3+5O2→4NO+6H2O. How much excess reactant remains after the reaction has stopped ( knowing that NH3 is the excess reactant )?
Answers
Answer:
0.017 moles of ammonia remains after the reaction is stopped.
Explanation:
The reaction is:
4NH₃ + 5O₂ → 4NO + 6H₂O
The first step is to convert the mass to moles, of each reactant:
2 g . 1mol/ 17g = 0.117 moles of NH₃
4 g . 1mol /32g = 0.125 moles of O₂
Ammonia, states the question, is the excess reactant so we can confirm it,
5 moles of oxygen need 4 moles of ammonia to react (by stoichiometry)
Then, 0.125 moles of oxygen may react to (0.125 . 4) / 5 = 0.1 moles
As we have 0.117 moles of ammonia and we need 0.1 moles.
(0.117 - 0.1) = 0.017 moles remains after the reaction is completed.
If we convert the moles to mass we have:
0.017 mol . 17 g /1mol = 0.289 g
A compound containing 5.93% H and 94.07% O has a molecular mass of 34.02 g/mol determine the empirical and Molecular formula Of this compound
Answers
Answer:
7% 4)2(10
Explanation:
beacouse if you divide it you can get the answer
If 120 g of potassium chloride are mixed with 100 g of water at 80°C, how much will not dissolve?
Answers
Answer:
yes
Explanation:
How many grams of KCl will dissolve in 1 liter of H2O at 50 °C? 5. 58.0 g of K2Cr2O7 is added to 100 g H2O at. 0 °C. With constant stirring, to what temp-.
Na2SO4
How many oxygen stems are?
Answers
Answer:
Na2SO4 means: two moles sodium (45.98 g), one mole sulfur (32.06 g), and four moles oxygen (64.00 g) combine to form one mole of sodium sulfate (142.04 g).
Explanation:
Answer:
As we can see in the formulae, Na₂SO₄
We can see that 4 is the subscript of O and that means There are 4 atoms of oxygen.
Shontal compared some of the properties of a marble to a piece
of wood. She placed a marble and a piece of wood in a bucket filled
with water. Shontal observed the wood floating on top of the water,
but the marble sank to the bottom of the bucket. Which statement
best explains why the marble and piece of wood acted differently in
the water?
Answers
Answer:
I can't see your answer choices, but it would be something that related to density of the marble vs. wood.
Explanation:
Density is mass divided by volume. A marble with a greater density than water will sink, and wood with lower density than water will float.
what is the answer to N2+H2=NH3
Answers
Explanation:
This can be fixed by multiplying the product contacting nitrogen by 2. o The new chemical equation is N2 +H2 →2 NH3. Reactants. Products. Nitrogen. 2. 2.
can someone explain in detail how molar mass, Avogadro's number, and volume are all connected through moles? Im so confused :(
Answers
Answer:
See Explanation
Explanation:
By definition, 1 mole is the mass of substance (or, formula mass in grams) containing 1 Avogadro's Number (N₀ = 6.02 x 10²³) of particles. That is ...
1 mole of hydrogen atoms (H) = 1.00794 grams
1 mole of molecular hydrogen (H₂) = 2.01588 grams
1 mole of any substance = 1 formula weight in grams
1 mole = 1 Avogadro's Number (N₀) = 1 formula weight in grams
In the concept of 'gas laws' 1 mole of any (all) gas at STP conditions ( => 0°C & 1 atmosphere pressure) occupies 22.4 Liters & is known as the 'molar volume' of a gas at STP. If the temperature &/or pressure change the volume will not be 22.4 Liters.
For reactions whose coefficients are balanced to the lowest whole number values (i.e., no fractional coefficients) the equation is known as the 'standard reaction' and conditions are assumed to be STP and the coefficients of gas phase components indicate molar volumes. Example ...
Given N₂(g) + 3H₂(g) => 2NH₃(g) is assumed to be at 0°C; 1 Atm pressure.
Molecular Nitrogen = 1 molar volume = 22.4 Liters of N₂(g)
Molecular Hydrogen = 3 molar volumes = 3 x 22.4 Liters of H₂(g) = 67.2 Liters of H₂(g)
Molecular Ammonia = 2 molar volumes = 2 x 22.4 Liters of NH₃(g) = 44.8 Liters of NH₃
_________deep-sea currents that occur because of differences between the salinity and temperature characteristics of water masses
Lesson 2.12
Question 3 options:
Density
Thermohaline currents
Salinity
Polar ice caps
Answers
Answer:
its B) Thermohaline currents Sorry if its wrong but im sure this could be it
Explanation:These deep-ocean currents are driven by differences in the water's density, which is controlled by temperature (thermo) and salinity (haline). This process is known as thermohaline circulation. In the Earth's polar regions ocean water gets very cold, forming sea ice.
Thermohaline currents deep-sea currents that occur because of differences between the salinity and temperature characteristics of water masses
What is thermohaline currents?Thermohaline currents is also known as 'the ocean conveyor belt' or 'the global conveyor belt'. It is a part of the large scale ocean circulations.
As the name thermohaline suggested that, 'thermo' means heat and 'haline' refers to the salt content. So, when there is a great difference in the temperature and the salt content deep sea currents occur.
Hence option (2) is correct i.e. thermohaline currents occur due to temperature and salinity difference.
To learn more about thermohaline currents, visit below link:
https://brainly.com/question/21899024
To investigate the effect of salt water on a cell, a researcher places one cell sample in freshwater and a second cell sample in salt water. After six hours, the researcher observes and sketches the two cells as shown. What change in the cells occurred during the six hours the cells remained undisturbed? A.Osmosis occurred in the salt water cell sample because the water from inside the cell moved out of the cell to a lower concentration, causing the cell to shrink. B.Osmosis occurred in the freshwater cell sample because the water from outside the cell moved into the cell to a higher concentration, causing the cell to grow. C.Osmosis occurred in the salt water cell sample because the salt from outside the cell moved into the cell to a lower concentration, causing the cell to shrink. D.Osmosis occurred in the freshwater cell sample because the salt from inside the cell moved out of the cell to a higher concentration, causing the cell to grow.
Answers
Answer:
MARK ME BRAINLIEST
Explanation:
MARK ME BRAINLIEST
The effective nuclear charge of a neutral atom aluminum, Al with atomic number 13 is
Answers
Answer:
wala hindi ko yan alam ty
The process of cell division in which the resulting cells have half the chromosomes number of the parent cell.
Question 1 options:
ossmosis
mitosis
meiosis
diffusion
Answers
Answer:
Meiosis
Explanation:
This is The process of cell division in which the resulting cells have half the chromosomes number of the parent cell.
Which statement about the organisms whose fossils are found in the rock is correct?
A.) Organism W existed much before Organism Y.
B.) Organism Y existed over the longest period of time.
C.) Organism Y and Organism U were alive over the same time span.
D.) Organism W existed over a shorter period of time than did Organism X. The table below shows the fossils of organisms found in various layers of an undisturbed rock:
Answers
Answer:
a?
Explanation:cause if organism w is a fossil and organism y is a rock the rock is way older than a fossil so a
Answer:
the answer is C
Explanation:
just did the test and Y, and U are in the same layer...so both were alive over the same time span.
3.3 mol Fe(OH)3 and 6.3 mole H2SO4react according to the equation 2Fe(OH)3 + 3H2SO4 → Fe2(SO4)3 + 6H2O.
If the limiting reactant is Fe(OH)3, determine the amount of excess reactant that remains. Answer in units of mol.
Answers
Answer and Explanation:
2 mol of Fe(OH)3 produces 1 mol of Fe2(SO4)3 then 2.1 mol of Fe(OH)3 will produce (1)/(2) x 2.01 = 1.05 mol of Fe2(SO4)3 amount of H2O = (6)/(2) * 2.01 = 6.03 mol HO2
If the atomic number of an element is 8 and the mass number is 16, the number of neutrons in an atom of that element is what? (show work)
Answers
Answer:
8.
Explanation:
Atomic number tells us the number of protons in an element. Mass number is the total amount of mass in an atom, and it comes from the sum of the mass of protons and neutrons, which are both considered to have a mass of 1 amu.
If the total mass is 16amu and 8amu comes from the 8 protons, the remaining mass comes from neutrons.
16 - 8 = 8
Nitrogen gas reacts with hydrogen gas to produce ammonia according to the following equation.
N2(g)+3H2(g) → 2NH3(g)
What is the mole of ammonia produced from 7.23 x 10−4 moles of the hydrogen reactant, assuming there is sufficient nitrogen to react?
7.23 x 10−47.23 x 10−4
8.42 x 10−38.42 x 10−3
4.82 x 10−44.82 x 10−4
4.83 x 10−3
Answers
Answer:
4.82 x 10^-4
Explanation:
7.23 x 10^-4 mol H2 l 2 mol NH3
------------------------------------------------------- =
l 3 mol H2
(7.23 x 10^-4) x 2 / 3 =
4.82 x 10^-4
Hope this helped! ;)
The mole of ammonia produced from 7.23 x 10⁻⁴ moles of the hydrogen reactant when there is sufficient nitrogen to react is 4.82×10⁻⁴.
What is moles?Moles is a unit which is used to estimate the amount of any substance and it is represented as:
n = W/M , where
W = given mass
M = molar mass
Given chemical reaction is:
N₂(g) + 3H₂(g) → 2NH₃(g)
In the question it is given that sufficient moles of N₂ is present, so the formation of product depends on the moles of H₂. From the stoichiometry of the reaction, it is clear that:
3 moles of H₂ = produce 2 moles of NH₃
7.23×10⁻⁴ moles of H₂ = produce 2/3 × 7.23×10⁻⁴=4.82×10⁻⁴ moles of NH₃
Hence, option (C) is correct i.e. 4.82×10⁻⁴.
To know more about moles, visit the below link:
https://brainly.com/question/1464305
Need help with the balancing one
Answers
is lime flavor ionic or covalent
Answers
Answer:Calcium carbonate (CaCO3), essentially, is an ionic compound having the bivalent calcium and carbonate ions. But the carbonate anion is a polyatomic species. The carbon atom is bonded to all the three oxygen atoms by covalent bonds - two carbon-oxygen single bonds and one carbon-oxygen double bond.
Explanation:So it's ionic hope this helps u. Btw may i have brainlist plz.
What is the specific heat capacity of an unknown metal if 75.00 g of the metal absorbs 418.6J of heat and the temperature rises 25.0°C?
Answers
Answer:
The specific heat capacity of the unknown metal is 0.223 [tex]\frac{J}{g*C}[/tex]
Explanation:
Calorimetry is the measurement and calculation of the amounts of heat exchanged by a body or a system.
There is a direct proportional relationship between heat and temperature. The constant of proportionality depends on the substance that constitutes the body as on its mass, and is the product of the specific heat by the mass of the body. So, the equation that allows calculating heat exchanges is:
Q = c * m * ΔT
where Q is the heat exchanged by a body of mass m, made up of a specific heat substance c and where ΔT is the temperature variation.
In this case, you know:
Q= 418.6 J c= ? m= 75 g ΔT= 25 CReplacing:
418.6 J= c* 75 g* 25 C
Solving:
[tex]c=\frac{418.6 J}{75 g*25 C}[/tex]
c= 0.223 [tex]\frac{J}{g*C}[/tex]
The specific heat capacity of the unknown metal is 0.223 [tex]\frac{J}{g*C}[/tex]
When thermal energy is removed from matter, what happens to the molecules?
Answers
Answer:
Figure it out.
Explanation:
Ms. Gratz went outside last night and saw a full moon as shown:
Which moon phase would she see next?
Answers
Nitric monoxide (NO) reacts with oxygen gas to form nitrogen dioxide (NO₂), a dark brown gas. If 5.895 mol of NO is mixed with 2.503 mol of O₂,
determine the limiting reagent.
calculate the number of grams of NO₂ produced.
and determine how many grams of excess reagent remain unreacted.
Answers
Answer:
Limiting reactant: O2
grams NO2 produced = 230.276 g NO2
grams of NO unused = 26.67 gNO
Explanation:
2NO + O2 --> 2NO2
Step 1: Determine the molar ratio NO:O2
molar ratio NO:O2 = 5.895: 2.503 = 2.35
stoichiometric molar ratio NO:O2 = 2:1
So, O2 is the limiting reactant.
Step2: Determine the grams of NO2:
?g NO2 = moles O2 x (2moles NO2/1 mol O2) x (MM NO2/ 1 mol NO2) = 2.503 x 2 x 46 = 230.276 g NO2
Step 3: Determine the amount of excess reagent unreacted
moles excess NO reacted = moles O2 x (2 moles NO/1 mol O2) = 2.503 x 2 = 5.006 moles NO reacted
moles NO unreacted = total moles NO - moles NO reacted = 5.895-5.006 =0.889 moles NO unreacted
mass NO unreacted = moles NO unreacted x MM NO = 0.889 x 30 =26.67 g NO unreacted
What is a disadvantage for reasons undergoing asexual reproduction
Answers
C1 Progress quiz: Atomic structure 2 - test
4 The atomic radius of a silicon atom is 1.1 x 10-10 m. Calculate its atomic radius in
nanometres.
O
11 nm
o
0.011 nm
0.11 nm
1.1 nm
Answers
Answer:
0.11 nm
Explanation:
1.1 x 10-10 m
The goal is to convert the atomic radius from meters (m) to nanometres (nm). We do this by multiplying the value by 10^9.
This is given as;
[tex]1.1 * 10^{-10} * 10^{9}\\1.1 * 10^{-10 + 9}\\1.1 * 10^{-1}\\0.11[/tex]
2. Combine lead (II) nitrate and potassium iodide solutions.
Pb(NO3)2+ Kl →
2. Combine lead (II) nitrate and potassium iodide solutions.
Pb(NO3)2+ Kl →
3. Combine magnesium metal and hydrochloric acid solution.
Mg + HCl →
4. Electrolysis (splitting) of water.
H2O →
5. Burning magnesium.
Mg + O2 →
Answers
Answer:
Pb(NO3)2+ 2Kl →PbI2 + 2KNO3
Mg + 2HCl → MgCl2 + H2
4H2O → 4H^+ + 4OH^-
2Mg + O2 → 2MgO
Explanation:
This question has to do with the balancing of chemical reaction equations. The general rule for balancing chemical reaction equation is that the number of atoms of each element on the left hand side of the reaction equation must be equal to the number of atoms of the same element on the right hand side of the reaction equation.
This principle was followed in balancing each reaction equation above. For instance, in the burning of magnesium, there are two atoms of both magnesium and oxygen on either side of the reaction equation.
I will give any one Brainliest, Thanks, & a 5 rating who can Guess my Top 2 Phobia's.
I will give 1 hint for each though...
1st phobia hint is we creep & crawl everywhere we may be small but some people get scared.
2nd phobia hint is things that life is gone and no longer breathes
Answers
Answer:
scared of bugs and dead things, or death itself?
Answer:
scared of bugs and dead things, or death itself?
Explanation:
All human body systems works together m. What would happen to someone if one of their systems becomes inefficient?
Answers
Answer:
lol this is what you get for stealing my points
Explanation:
What is an example of quantity of the resource being used by humans that affected
Answers
Answer:
1. Healthy and well educated people contribute to their societies in positive way
2. Malnourished and illiterate people cannot contribute much to their respective societies.
Explanation:
PLSSS HELP ANYONE ASAP!
Answers
Round the following number to
3 significant figures:
3,545,530
Answers
The concept of significant figures are mainly used by scientist and engineer to know the significance of digits in a measurement. Therefore, 3,545,530 upto 3 significant figures is 3.54 x 10³.
What is significant figures?Significant figures are the figures that indicate the degree of accuracy of a value. It tells about the precision of a value. It gives an idea about the digits that are necessary to indicate the experimental value.
Rules for counting significant figures are:
Number between 1 to 9 is always significant
Zeroes after a number has got no significance
Zeroes before a number has got no significance
Zeroes between number has got significance
3,545,530 upto 3 significant figures is 3.54 x 10³.
Therefore, 3,545,530 upto 3 significant figures is 3.54 x 10³.
To learn more about Significant figures, here:
https://brainly.com/question/12656148?
#SPJ1