Answers
Answer:
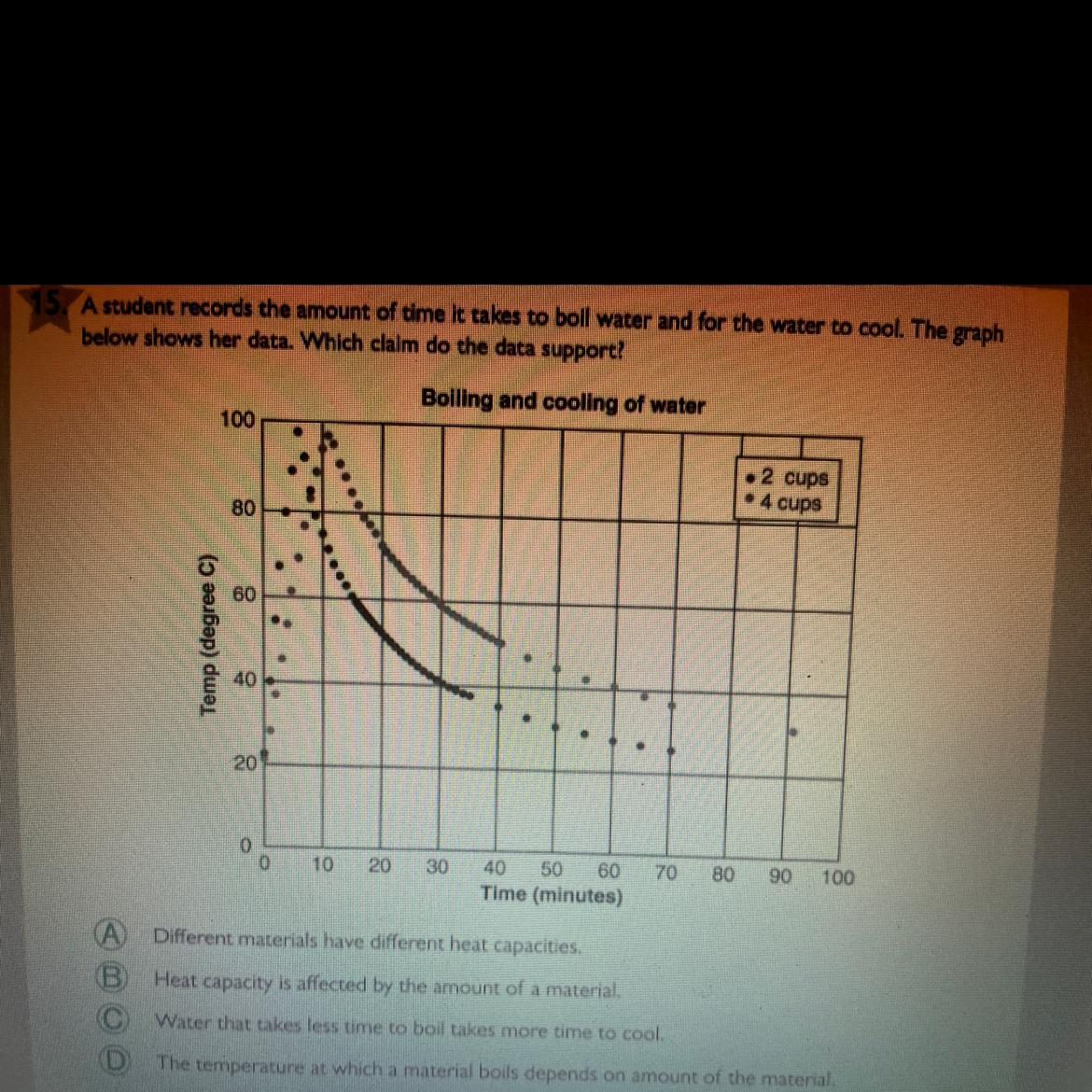
Answer is B
Explanation:
It's not A because we're boiling only water.
It's not C because the last dot for 2 cups ends at 70 min while the last dot for 4 cups ends at 90 min.
It's not D because water boils at around 100 C, as shown in the graph. The highest dot for both 2 cups and 4 cups were about the same near 100 C.
The graph shows that 4 cups of water is able to retain heat more (higher heat capacity), so it takes more time for water to cool down than 2 cups of water.
Related Questions
What is the mass of 500 trillion (5.0 x 10'4)
molecules of water?
Answers
Pls help me over here
Answers
Answer:
1 and the element is hydrogen (there is an exception for the octet rule for the element hydrogen) hope this helps
Explanation:
What is the correct shape and polarity of a water molecule
Answers
Answer:
D
Explanation:
because both H's should be positive and O is supposed to be negative.
A burning match will burn more vigorously in pure oxygen than in air because _________ . Select one: a. oxygen is a catalyst for combustion b. nitrogen is a reactant in combustion and its low concentration in pure oxygen catalyzes the combustion c. oxygen is a product of combustion d. nitrogen is a product of combustion and the system reaches equilibrium at a lower temperature e. oxygen is a reactant in combustion and pure oxygen increases the reactant concentration
Answers
Answer:
e. oxygen is a reactant in combustion and pure oxygen increases the reactant concentration
Explanation:
The reaction of a burning match is combustion. In this combustion, the organic components of the match (such as cellulose, C₆H₁₀O₅) react with oxygen, producing water and carbon dioxide:
C₆H₁₀O₅(s) + 6O₂(g) → 5H₂O(g) + 6CO₂(g)Seeing as oxygen is a reactant and not a catalyst nor product, and that nitrogen plays no part in the reaction, the only correct answer is option e.
Choose a reasonable explanation to account for the differences. There may be more than one possible reason that makes sense, just select one of them.
A. It is possible not all of the water was evaporated from the sand, causing the recovered mass to be higher.
B. It is possible not all of the water was evaporated from the sand, causing the recovered mass to be lower.
C. While drying the NaCl, the liquid boiled and some splattered out of the evaporating dish, causing the recovered mass to be lower
D. While drying the NaCl, the liquid boiled and some splattered out of the evaporating dish, causing the recovered mass to be higher.
E. There was no difference in recovered and original mass, so there is no difference to account for.
Answers
Answer:
A. It is possible not all of the water was evaporated from the sand, causing the recovered mass to be higher
D. While drying the NaCl, the liquid boiled and some splattered out of the evaporating dish, causing the recovered mass to be higher.
Explanation:
Sand absorbs water and stores it. The sunlight causes the water to evaporate but sand can hold some of the water inside it. This results in increase in mass of the sand. The mass of sand before and after the water evaporation can be different.
Determine if the following two structures are
identical, isomers, or unrelated?
A
B
С
identical
isomers
unrelated
Answers
Answer:
its C
Explanation:
The skeletal structure shows the outline of the compound with lines and bonds. Both the structures are unrelated. Thus, option C is correct.
What are isomers?Isomers are structures with identical molecular structures but differ in the skeletal or molecular representation. They also have varied properties as compared to the parent structure.
Identical compounds are structures that have the same atoms and are arranged with the same spatial design and orientation. The number of atoms and the spatial arrangement of the two compounds is different.
Therefore, the two compounds are unrelated.
Learn more about isomers here:
https://brainly.com/question/26658261
#SPJ2
How many moles are in 2.5L of 1.75 M Na2CO3
Answers
Answer:
4.375 moles Na2CO3
Explanation:
Formula for molarity is;
M = n/V
Where;
M is molarity
n is number of moles
V is volume
Making n the subject we have;
n = VM
We are given;
M = 1.75 M
V = 2.5 L
Thus;
n = 2.5 × 1.75
n = 4.375 moles Na2CO3
Answer:
4.375 moles Na2CO3
Explanation:
Have a great day
All forms of energy can be traced back to
Answers
Calculate the moles of electrons obtained from 250coulomb of electricity
Answers
Answer:
[tex]n=0.00259 mol[/tex]
Explanation:
Hello there!
In this case, since the Faraday's constant is 96500 Coulombs per mole, we can set up the following process to obtain the moles in 250 Coulombs:
[tex]n=\frac{250C}{96500C/mol}\\\\n=0.00259 mol[/tex]
Best regards!
I need help plzzzzzz
Answers
I hope this helps
Answer:
it is 25%
Explanation:
Answering so you can give the other person brainliest :D have a good day
Which of these statements best supports the idea that a cell is the basic unit of a living organism?
A.
The number of cells in an organism affects the size of that organism.
B.
A tissue is composed of cells with similar structure and function.
C.
Some organisms have only one cell.
D.
All organisms are made up of one or more cells.
Answers
Answer:
The answer is D.
Explanation:
I searched it up :)
After mixing for three hours, the product is extracted into dichloromethane and the solvent is removed to give 245 mg of an oil. Using the moles of our protected aldehyde calculated earlier (2.96) and the molecular weight of the product (102 g/mol) predict the theoretical 100% yield of the product in milligrams. Round to the tenths place.
Answers
Answer:
the theoretical 100% yield of the product in milligrams is 302920 mg
Explanation:
Given the data in the question;
245 mg of an oil
Using the moles of our protected aldehyde calculated earlier (2.96)
the molecular weight of the product (102 g/mol) = 102000 mg/mol
so, the mass of aldehyde produced (100% yield) will be;
⇒ number of moles × molar mass
⇒ 2.96 mol × 102000 mg/mol
⇒ 302920 mol.mg / mol
⇒ 302920 mg
Therefore, the theoretical 100% yield of the product in milligrams is 302920 mg
Helpppp!!!
Which Advantages do instrumental methods of chemical analysis have over traditional methods?
A. Accuracy, Sensitivity, identification, and automation
B. Accuracy, sensitivity, outsourcing, and automation
C. Accuracy, analysis, rapidness, and automation
D. Accuracy, sensitivity, rapidness, and expertise
E. Accuracy, sensitivity, outsourcing, and automation
Answers
Answer:
your answer would be E
Explanation:
I took that class
Titanium(IV) chloride decomposes to form titanium and chlorine, like this:_____.
TiCl4(1)-→Ti(s) + 2 Cl 2(g)
At a certain temperature, a chemist finds that a reaction vessel containing a mixture of titanium(IV) chloride, titanium, and chlorine at equilibrium has the following composition:_____.
compound amount
TiCl4 4.18g
Ti 1.32g
Cl2 1.08g
Calculate the value of the equilibrium constant for this reaction. Round your answer to significant digits. Clears your work. Undoes your last action. Provides information about entering answers.
Answers
Answer:
K = 8.6x10⁻⁶
Explanation:
a chemist finds that a 5.2L reaction vessel...
To solve this question we need first to find the equation of the equilibrium constant using the chemical eqiation:
TiCl₄(l) ⇄ Ti(s) + 2Cl₂(g)
The equilibrium constant expression is:
K = [Cl₂]²
Because equilibrium constant is defined as the ratio berween concentrationa of products over reactant powered to its reaction coefficient. But pure liquids as TiCl₄(l) and pure solids as Ti(s) are not taken into account
Now, we need to find the molar concentration of Cl₂, [Cl₂]:
Moles Cl₂ -Molar mass: 70.9g/mol-:
1.08g * (1mol / 70.9g) = 0.0152 moles / 5.2L =
2.93x10⁻³M = [Cl₂]
K = (2.93x10⁻³)²
K = 8.6x10⁻⁶PLEASE ANSWER!! THANK YA!
Using Graham's Law of Effusion, calculate
the approximate time it would take for
1.0 L of argon gas to effuse, if 1.0 L of
oxygen gas took 12.7 minutes to effuse
through the same opening.
Answers
Answer:
I think the answer is X
Explanation:
X is a variable and variables stand for the unknown
chemical properties of elements
Answers
Answer:
Atomic number. The atomic number indicates the number of protons within the core of an atom. ...
Atomic mass. The name indicates the mass of an atom, expressed in atomic mass units (amu). ...
Electronegativity according to Pauling. let me know if you need more ;)
g The alkali metals are able to displace hydrogen readily from water. The alkaline earth metals are able to do so as well, although not nearly as vigorously. They are easily able to displace hydrogen from acid. a: Write and balance the equation for any of the alkali metals (pick your favorite!) reacting with water to form hydrogen gas and the metal's hydroxide (e.g. sodium hydroxide, potassium hydroxide). (10 points) b: Write and balance the equation for any of the alkaline earth metals (the second column on the left) reacting with hydrochloric acid to form hydrogen gas and the metal's chloride salt (e.g. magnesium chloride, calcium chloride) (10 points)
Answers
Answer:
See explanation
Explanation:
In writing a chemical reaction equation, we must ensure that it is balanced. In a balanced chemical reaction equation, the number of atoms of each element on the left hand side of the reaction equation must be equal to the number of atoms of the same atom on the right hand side of the reaction equation.
Let us now consider the reaction of NaOH with water as follows;
2Na(s) + 2H2O(l) ------> 2NaOH(aq) + H2(g)
For the reaction of Magnesium metal and HCl we have;
Mg(s) + 2HCl(aq) -------> MgCl2(aq) + H2(g)
Please please help me please please help please please help me please please help please please help me
Answers
Answer:
AU9NJ-BLVHV-TCLJS-54YTB
Photosynthesis needs water and carbon dioxide to happen. These 2 ingredients are called _______.
Answers
Please help I’ll mark brainliest :)
Answers
Answer:
Biotechnology is the use of living organisms through modification to make products of technological or industrial significance.
positively, biotechnology can be used in manufacture of medicine and also synthetic forms of various hormones for use in the health and medical field.
negatively biotechnology may be manipulated for use in wars as biological warfare that can lead to loss of lives and environmental degradation
Explanation:
Answer: Biotechnology is the use of living organisms through modification to make products of technological or industrial significance.
positively, biotechnology can be used in manufacture of medicine and also synthetic forms of various hormones for use in the health and medical field.
negatively biotechnology may be manipulated for use in wars as biological warfare that can lead to loss of lives and environmental degradation
The iodide (I2) content of a commercial mineral water was measured by two methods that produced wildly different results.6 Method A found 0.23 milligrams of I2 per liter (mg/L) and method B found 0.009 mg/L. When Mn21 was added to the water, the I2 content found by method A increased each time that more Mn21 was added, but results from method B were unchanged. Which of the Terms to Understand describes what is occurring in these measure- ments? Which result is more reliable?
Answers
Answer:
Results from method B is more reliable than method A.
Explanation:
The two method that are used for the analysis produced different results. The first method that is method A gives higher value of the iodine content than the method B.
When [tex]$Mn^{2+}$[/tex] was added to water, method A showed an increased in the iodine content and it increases with the increase in the amount of [tex]$Mn^{2+}$[/tex] .
Where as in the method B, there is no change in the results. Therefore the measurements provided by the method A shows an inference of [tex]$Mn^{2+}$[/tex] ion.
The measurement of the iodine content is affected by the presence of the ion [tex]$Mn^{2+}$[/tex] in water.
Since in method B there is no change in measurement, it is independent of the presence [tex]$Mn^{2+}$[/tex] ion in water.
As higher iodine content is given by method A, so [tex]$Mn^{2+}$[/tex] ion must be present in original water that must be interfering the measurement. Hence, method B is more reliable.
Method B is more reliable as its value is not altered by external factors such as contamination.
What is water analysis?Water analysis is done to test the quality of water.
The method employed in water analysis is titrimetric analysis.
In the test iodide (I⁻) content of a commercial mineral water using the two methods, the results of the test showed different values for the iodide content.
However, since addition of Mn²⁺ to the mineral water increases the iodide content value from Method A while that of Method B remains unchanged, Method B is more reliable as its value is not altered by external factors such as contamination.
Learn more about titrimetric analysis at: https://brainly.com/question/8463543
When a hydrocarbon fuel is burned, almost all of the carbon in the fuel burns completely to form CO2 (carbon dioxide), which is the principal gas causing the greenhouse 102 ENERGY, ENERGY TRANSFER effect and thus global climate change. On average, 0.59 kg of CO2 is produced for each kWh of electricity generated from a power plant that burns natural gas. A typical new household refrigerator uses about 700 kWh of electricity per year. Determine the amount of CO2 production that is due to the refrigerators in a city with 300,000 households.
Answers
Answer:
1.239 * 10^8 Kg
Explanation:
Since all the electricity consumed comes from natural gas;
amount of electricity consumed = 700 * 300,000 = 2.1 * 10^8 kWh
So, amount of CO2 consumed is given by;
amount of electricity consumed * amount of CO2 per kWh
Hence,
Amount of CO2 = 2.1 * 10^8 kWh * 0.59 = 1.239 * 10^8 Kg
Convert 23.92 mm to meter
Answers
Answer:
0.02392 m
Explanation:
Kilo - Hecto - Deka - Meters/Liters/Grams - Deci - Centi - Milli
23.92 mm ---> 0.02392 m
Basically move the decimal 3 units to the left.
Hope this helps and stay safe, happy, and healthy, thank you :) !!
What is the molar mass of H2CO3?
(Molar mass of H = 1.0079 g/mol; C = 12.010 g/mol; O = 15.999 g/mol)
29.02 g/mol
46.04 g/mol
62.02 g/mol
72.08 g/mol
Answers
Answer:
62.02 g/mol
62.03 g/mol
Explanation:
62.03 g/mol
The molar mass of H2CO3 is 62.02 g/mol
What is molar mass?The molar mass of the compound is the sum of molar mass of indivitual atom multiple by number of that atom.
Calculation of molar mass of H2CO3 ,
Molar mass of H2CO3 = Molar mass of H*2 + Molar mass of C + Molar mass of O*3
Molar mass of H2CO3 = 1.0079*2 + 12.010 + 15.999*3
Molar mass of H2CO3 = 2.0158 + 12.010 + 47.997
Molar mass of H2CO3 = 62.02 g/mol
To learn more about Molar mass here.
https://brainly.com/question/12127540
#SPJ3
Which states have no oil or gas production?
Answers
Answer:
Georgia and minessota
Explanation:
they have no production
Minnesota and Georgia.
Hope this helps! :)
How many molecules are there in 4.3 moles of calcium sulphate ?
Answers
Answer:
The law companies that value excessive ethics comply with the legal guidelines not solely in letter however go past what is stipulated 1 My proudest accomplishment is organizing a donation pressure aimed at helping the less fortunate humans skills
Explanation:
My bedroom is very clean and big closest it color
A chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the door of the cabinet says the cabinet is used to store bottles of carbon tetrachloride, diethylamine, methyl acetate, ethanolamine, and dimethyl sulfoxide.
The chemist plans to try to identify the unknown liquid by measuring the density and comparing to known densities. First, from his collection of Material Safety Data Sheets (MSDS), the chemist finds the following information:
Liquid Density
Tetrahydrofuran 0.89·gcm^−3
Carbon tetrachloride 1.6·gcm^−3
Pentane 0.63·gcm^−3
Dimethyl sulfoxide 1.1·gcm^−3
Acetone 0.79·gcm^−3.
Next, the chemist measures the volume of the unknown liquid as 0.852L and the mass of the unknown liquid as 938.g . Calculate the density of the liquid.
Answers
Answer: The density of the liquid is [tex]1.10g/cm^3[/tex]
Explanation:
Density is defined as the mass contained per unit volume.
[tex]Density=\frac{mass}{Volume}[/tex]
Given : Mass of the unknown liquid = 938 grams
Volume of the unknown liquid = [tex]0.852L=852cm^3[/tex] [tex](1L=1000cm^3[/tex]
Putting in the values we get:
[tex]Density=\frac{938g}{852cm^3}[/tex]
[tex]Density=1.10g/cm^3[/tex]
Thus the density of the liquid is [tex]1.10g/cm^3[/tex] and the liquid is dimethyl sulfoxide.
hi, if your looking for extra points (50+) and br ainiest here is ur chance, answer this question correctly plz
Answers
Think about a hot summer afternoon at the beach
or a lake. How do the temperatures of the sand
and water compare?
A. The sand is hot; the water is cool.
B. The sand is hot; the water is hot.
C. The sand is cool; the water is cool.
D. The sand is cool; the water is hot.
Answers
Answer:
D
Explanation:
I say this because of this that I read. There was a study shown
"Our experiment was a success. We obtained data that answered our problem, “Which substance absorbs the most heat on a hot day? Sand, water, or sand with water?” The results showed that from the twenty-five trials: the cup of sand temperature ranged from 63-68°C, cup of water temperature ranged from 71-77°C, and the cup of sand with water temperature ranged from 66-71°C. Based on our data, our hypothesis was incorrect. We hypothesized that the sand would absorb the most heat since it is darker and less reflective than water; but from our data, water absorbed the most heat. In conclusion, sand absorbed the least amount of heat and water absorbed the most. Next time we want to cool down at the beach, we’ll know where to go!"
This was an experiment that was performed and this is the conclusion
Hope that helps
A compound contains 0.5 mol Na, 0.5 mol N, and 10 mol H. The empirical formula of the
compound is -
Answers
Answer:
NaNH₂₀
Explanation:
0.5 mol Na, 0.5 mol N, and 10 mol H
To obtain the empirical formulae, we find the mole ratio between the elements and this is done by dividing all through by the smallest mol (0.5)
Na = 0.5 / 0.5 = 1
N = 0.5 / 0.5 = 1
H = 10 / 0.5 = 20
The mole ratio is used to write the empirical formulae. It is given as;
NaNH₂₀