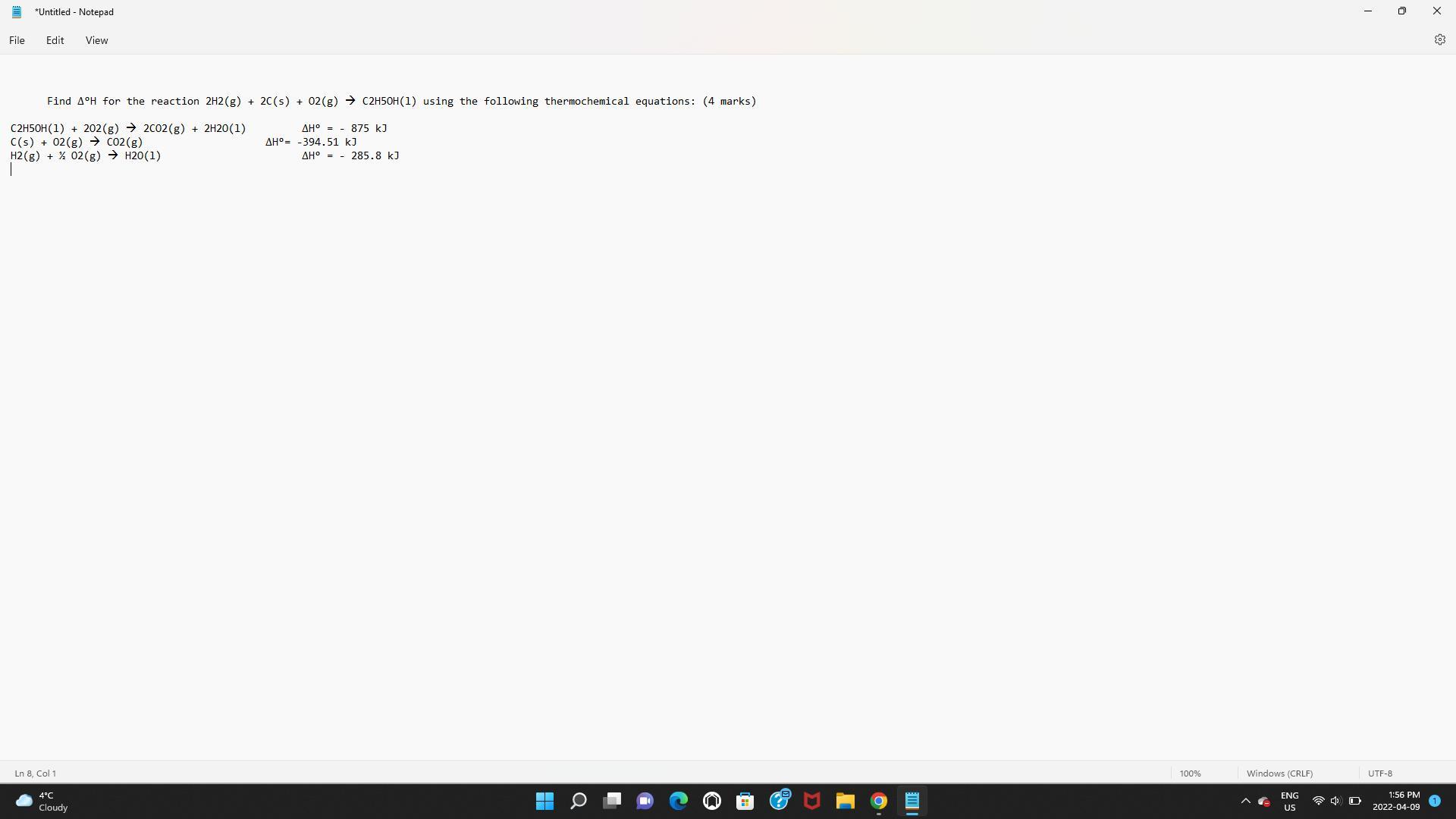
Answers
Answer:
Explanation:
Here, we want to get the ∆°H for the reaction
Mathematically, to get this, we have to subtract the change in enthalpy of the reactants from that of the product
However, we shall not be considering the enthalpy values for single element molecules like hydrogen molecule and oxygen molecule
From the later reactions and values given, we can get the values for carbon (iv) oxide and Ethanol
For Carbon (iv) oxide, we have it as:
[tex]\begin{gathered} \text{ enthalpy of product - enthalpy of reactant =-394.5 KJ} \\ enthalpy\text{ of reactant here is zero} \\ \text{ Thus enthalpy of product CO}_2\text{ = -394.51 KJ} \end{gathered}[/tex]For Ethanol, we need to get the value for water
We have that as follows:
[tex]-285.8\text{ KJ}[/tex]For ethanol, that would be: Let us label it e
[tex]undefined[/tex]Related Questions
How will you describe the pattern as electrons are distributed
Answers
How will you describe the pattern as electrons are distributed?
In a simple way, you can say, electrons are arranged around an atom's nucleus.
When electrons are near the nucleus, have the lowest energy. Electrons further aways will have higher energy.
We can say electrons are in different shells with different energy. Inside those shells, electrons are in subshells too.
Then you have some numbers that describe the completly characteristics of an electron in an atom.
They are called Quantum Numbers. They are four
1) The principal Quantum number (n): describes the energy of an electron and the most probable distance of the electron from the nucleus. In other words, it refers to the size of the orbital and the energy level an electron is placed in.
2) The number of subshells or (l): describes the shape of the orbital. It can also be used to determine the number of angular nodes.
3) The magnetic Quantum number (ml): describes the energy levels in a subshell. (Remember we have shells and inside of them we have subshells)
4) The Spin of the Electron (ms): refers to the spin of the electron which can either be up or down.
[tex]_{m_{s\text{ }}}[/tex](a) From the differential rate equation given below, write a balanced equation for the reaction involved. 1 d[N o,) i d[NO] d[0] N2011 2 2 dt 4 dt dt rate (b) Given, rate = k[NO2]2[Cl2], by what factor does the rate increase of each of the following changes occurs. i. (NO2) is tripled. ii. [NO2] and [Cl2] are doubled [4M]
Answers
We are given an equation that shows the differential rate change of the reaction and it is given as
[tex]-\frac{1}{2}\frac{d}{dt}\lbrack N_2O_5\rbrack=\frac{1}{4}\frac{d}{dt}\lbrack NO_2\rbrack=\frac{d}{dt}\lbrack O_2\rbrack[/tex]This depicts the decomposition reaction of N₂O₅. The forward reaction (product) carries a positive sign while the backward reaction or (reactant) carries a negative sign.
We can as well integrate this, but for the sake of simplicity, the equation of reaction is given as
[tex]2N_2O_5\rightleftarrows4NO_2+O_2[/tex]Which of the following results in a new substance being formed?-Physical Change-Physical Property-Chemical Change-Chemical Property
Answers
Answer
Chemical change
Explanation
Chemical changes occur when a substance combines with another to form a new substance,
Calculate the molarity of a solution that is made of 75 g NaCl in 1.0 L of water
Answers
Answer
Molarity = 1.28 mol/L
Explanation
Given:
Mass of NaCl = 75 g
Volume of water = 1.0 L
What to find:
The molarity of the solution.
Step-by-step solution:
The molarity of the solution can be calculated using the molarity formula given below:
[tex]Molarity=\frac{Mole}{Volume\text{ }in\text{ }L}[/tex]However, you need to convert the mass of NaCl given to moles using the mole formula; Mass divided by molar mass.
The molar mass of NaCl = 58.44 g/mol
[tex]Mole=\frac{Mass}{Molarmass}=\frac{75\text{ }g}{58.44\text{ }g\text{/}mol}=1.283367556\text{ }mol[/tex]Now, the molarity is
[tex]Molarity=\frac{1.283367556\text{ }mol}{1.0\text{ }L}=1.283367556\text{ }mol\text{/}L\approx1.28\text{ }mol\text{/}L[/tex]Hence, the molarity of the solution is 1.28 mol/L
convert 8 mol Na to grams
Answers
184g
Explanations:The formula for calculating the mass of a substance is expressed as;
[tex]moles=mass\times molar\text{ mass}[/tex]Given the following parameters
• Moles of Na = 8moles
,• Molar mass of Na = 23g/mol
Substitute the given parameter into the formula
[tex]\begin{gathered} Mass\text{ of Na}=moles\times molar\text{ mass} \\ Mass\text{ }of\text{ Na}=8moles\times\frac{23g}{mol} \\ Mass\text{ of Na}=184g \end{gathered}[/tex]Hence the mass of the sodium is 184g
5. 5. A 3 gram sample of Zinc is heated with 100 J and then placed in a calorimeter. Thetemperature of the Water increases from 22 C to 25 C. What is the Specific Heat of
Answers
Answer
The Specific Heat of zinc = 11.11 J/g°C
Explanation
Given:
Mass of the zinc sample, m = 3 g
Quantity of heat, Q = 100 J
Initial temperature, T₁ = 22 °C
Final temperature, T₂ = 25 °C
The change in temperature, ΔT = T₂ - T₁ = 25 °C - 22 °C = 3 °C
What to find:
The Specific Heat of zinc, c
Step-by-step solution:
The Specific Heat of zinc, c can be calculated using the formula given below.Q=m
[tex]\begin{gathered} Q=mc\Delta T \\ \\ \Rightarrow c=\frac{Q}{m\Delta T} \end{gathered}[/tex]Putting the values of the given parameters into the formula, we have;c
[tex]c=\frac{100\text{ }J}{3g\times3°C}=\frac{100J}{9\text{ }g°C}=11.11\text{ }J\text{/}g°C[/tex]Hence, the Specific Heat of zinc is 11.11 J/g°C
What is the average mass, in grams, of one atom of niobium? (NA = 6.022 × 1023 mol–1)
Answers
1) List known data
NA = 6.022*10^23
List unknown data
Mass in grams:
Molar mass: 92.906 g/mol
2) Find out the mass
[tex]gramsof1atomofNiobium=92.906\frac{g}{\text{mol}}\cdot\frac{1\text{mol of niobium}}{6.022\cdot10^{23}\text{atoms of niobium}}=1.543\cdot10^{-22}g[/tex]One atom of niobium weighs 1.543*10^-22 g
How many moles of hydrogen gas are present in a 50.0 L steel container if the pressure is at 10.0 ATM and the temperature is 27.0 C(R=.0821atm/mole-k)
Answers
• We are given the following :
P = 10 Atm
V= 50 L
R=0.0821atm/mole-k)
T= 27°C = 273.15 + 27°C = 300 .15 K = 300K
• We will use the following formula in order to find moles of hydrogen gas
PV = nRT
n = PV /RT
=( 10 * 50 )/ ( 0.0821 * 300)
n = 500/24.63
n = 20.3 moles
Therefore ; moles of hydrogen gas present = 20.3 moles
4. In a polar covalent bond, the bonded atoms.(1 Point)
Share the electrons equally
Share the electrons unequally.
One atom loses and the other gains.
Both atoms gain electrons
5. In a molecule of bromine Br2
the difference in electronegativity is zero and the bond is polar covalent
the difference in electronegativity is zero and the bond is ionic.
the difference in electronegativity is zero and the bond is nonpolar covalent.
Not enough information to tell.
6. As the difference in electronegativity increases the bond Tends to be
more ionic
less ionic
more covalent
not related
7. a covalent bond takes place between which of the following ?
Metal and a metal
Nonmetal and metal
Nonmetal and nonmetal
All elements of the periodic table
8. When the two atoms share a pair of electrons (one electron each) they have
Double bond
Triple bond
single bond
not enough information to tell
9. atoms try to have a configuration similar to that of a noble gas. This rule is called
. hund's rule
Aufbau principle
Rule of triple
Octet rule
10.1. Which is correct about element A whose electron configuration is 1S2/ 2S2 2P4 ?
Has 4 valence electrons and needs 2 to fulfil the octet rule.
Has 4 valence electrons and needs 4 to fulfil the octet rule.
Has 6 valence electrons and needs 2 to fulfil the octet rule.
Has 6 valence electrons and needs 6 to fulfil the octet rule
Answers
Answer for number 4: Share the electrons unequally (I think. I am not 100% about this answer)
Explanation: Covalent bonds in which atoms share electrons unequally are polar. Covalent bonds involve the sharing of electrons between two atoms, but those electrons are not always shared equally. As the electronegativity difference between atoms in a covalent bond increases, electron sharing becomes less even.
How many valence electrons do the alkaline earth metals have?A.8B.6C.2D.4
Answers
answer and explanation
alkali earth metals are on the second group of the periodic table and the have two valence electrons
option: C
Is this microwaveable
Answers
Answer:
I wouldn't risk it if i were you haha
Explanation:
Select the correct answer from each drop-down menu.
What causes atoms to form covalent bonds?
in a covalent bond, two atoms are heid together by the attraction between
The number of
covalent bonds that an atom can form depends on the number of
in the atom.
Answers
Hence, the number of covalent bonds that an atom can form depends on the number of in the atom is correct.
To know more about Covalent bond check the below link:
https://brainly.com/question/3447218
#SPJ9
What volume of hydrogen gas is evolved from a reaction between 0.52 g of Na and water? The gas is collected at 25°C and 740 mmHg.___ Na (s) + ___ H2O (l) → ___NaOH (aq) + ___ H2 (g)
Answers
0.284L
ExplanationsThe balanced chemical reaction between sodium and water is expressed as:
[tex]2Na(s)+2H_2O(l)\rightarrow2NaOH(aq)+H_2(g)[/tex]Determine the moles of sodium Na that reacted
[tex]\begin{gathered} moles\text{ of Na}=\frac{mass}{molar\text{ mass}} \\ moles\text{ of Na}=\frac{0.52}{23} \\ moles\text{ of Na}=0.0226moles \end{gathered}[/tex]Based on stoichiometry, 2moles of sodium produces 1 mole of hydrogen gas. The moles of hydrogen gas required is given as:
[tex]\begin{gathered} mole\text{ of H}_2=\frac{1}{2}\times0.0226 \\ mole\text{ of H}_2=0.0113moles \end{gathered}[/tex]According to the ideal gas equation
[tex]\begin{gathered} PV=nRT \\ V=\frac{nRT}{P} \end{gathered}[/tex]Given the following
P = 740mmHg = 0.974atm
T = 25 +273 = 298K
R = 0.08205 Latm/molK (Gas constant)
Substitute the given parameters into the formula to have:
[tex]\begin{gathered} V=\frac{0.0113\times0.08205\times298}{0.974} \\ V=\frac{0.2764}{0.974} \\ V=0.284L \end{gathered}[/tex]Hence the volume of hydrogen gas that evolved from the reaction is 0.284L
Calculate the final concentration of a solution created through the following process: 1.7L of a 2.4 M NaF solution is diluted with water to 3.3 L of NaD solution.
Answers
The final concentration when 1.7L of a 2.4 M NaF solution is diluted with water to 3.3 L is 1.24 M
Calculating final concentration of solution :
For calculating the final concentration when 1.7L of a 2.4 M NaF solution is diluted with water to 3.3 L we use below given formula
[tex]M_{1} V_{1}[/tex]= [tex]M_{2} V_{2}[/tex]
Where,
[tex]M_{1}[/tex]= 2.4 M
[tex]V_{1}[/tex]= 1.7 L
[tex]M_{2}[/tex]= 'X' to be calculated
[tex]V_{2}[/tex]= 3.3L
(2.4)*1.7=X*3.3
X= 2.4*1.7/3.3
X= 1.24 M
How to calculate concentration ?Divide the solute's mass from the total volume of the solution. Using m as the solute's mass and V as the total volume of the solution, write out the equation C = m/V.
For more information on concentration kindly visit to
https://brainly.com/question/14133486
#SPJ1
A sample of liquid ethanol is added to a sealed glass beaker.A sealed beaker contains liquid ethanol, represented as circles. Most of the circles are in the liquid, but a few are in the space above the liquid.Figure © 2019 StrongMindBeaker: BlueRingMedia/ShutterstockWhat will happen to the ethanol after a minute?
Answers
We don't have information about how fast the equilibrium will be reached, however, we can analyze the options to see which ones are wrong.
What in fact happens is that the system's previous conditions doesn't matter much. After it is sealed, there is no way something gets out. At first, the system will not be at an equilibrium, it needs some time for that.
The system will have ethanol gas condensating and liquid ethanol evaporating, but at different rates.
Assuming the rate of evaporation is higher at the beginning, we will get more ethanol gas with time, but this will decrease the rate of evaporation a situation where both rates are equal. After that point, the system will be at equilibrium, meaning the amount of liquid and gas won't change anymore.
Having that in mind, the first alternative have the first part wront because the system nees time to get to equilibrium, but its second part is wrong too, because as we said the system will eventually stop getting more ethanol gas.
The second is wrong because both evaporating and condensation are happening at the same time, not one after the other.
The third is wrong because it wil always have an equilibrium. If all ethanol condensates, the rate of condensation will be zero and any evaporation that happens will make some ethanol gas back.
We can't know for sure that 1 minute will be enough to reach the equilibrium, but assuming it is, the fourth alternative perfectly describes what is happening: ethanol condensates and increase the pressure up to a point where both rates equilizes and the system reach the equilibrium.
So, the correct option is the fourth alternative.
A person has a sample of compound which has a formula that is C4H9. What percent carbon by mass is the compound? *(Note this is not a real compound)*
Answers
The percent cabon in the compound is 84.22%.
1st) We need to calculate the molar mass of C4H9 with the atomic mass of carbon (C) and hydrogen (H). We can find the atomic mass in the Periodic Table of Elements:
- Carbon atomic mass: 12.01 g/mol
- Hydrogen atomic mass: 1 g/mol
The compound C4H9 has 4 carbon atoms and 9 hydrogen atoms:
(12.01 g/mol x 4) + (1 g/mol x 9) = 57.04 g/mol
So, compound C4H9 weighs 57.04g/mol.
2nd) We know that 57.04 g represents the 100% of C4H9, so with a mathematical Rule of Three we can calculate the percent carbon in C4H9 knowing that all the carbon in the molecule weighs 48.04g (12.04 g x 4):
[tex]\begin{gathered} 57.04g-100\% \\ 48.04g-x=\frac{48.04g\cdot100\%}{57.04g} \\ \\ x=84.22\% \end{gathered}[/tex]Finally, the percent cabon by mass in the compound is 84.22%.
A 10 L cylinder of gas is stored at room temperature (25 Celsius) and a pressure of 1800 psi. if the gas is transferred to a 6.0 L cylinder, at what temperature would it have to be stored in order for the pressure to remain at 1800 psi?
Answers
Since we want the pressure to remain constant we have to use Charles Law of gases, that relates the volume and the temperature at a constant pressure:
[tex]\frac{V1}{T1}=\frac{V2}{T2}[/tex]In this case, we know the values of V1, T1 and V2, which are 10L, 25°C and 6.0L. Using these values we have to find T2:
[tex]\begin{gathered} T2=\frac{V2\cdot T1}{V1} \\ T2=\frac{6.0L\cdot25}{10L} \\ T2=15 \end{gathered}[/tex]The gas has to be stored at 15°C.
1. from the mass of copper measured 51.226 , determine the moles of Cu
Answers
To determine the moles of copper we must use its atomic weight. This value can be found in the periodic table. The atomic weight of copper is 63.546g/mol, so the moles of copper will be:
[tex]\begin{gathered} \text{Moles Cu= Given g of Cu }\times\frac{1molCu}{Atomic\text{ weight Cu, gCu}} \\ \text{Moles Cu=51.226gCu}\times\frac{1molCu}{63.546\text{gCu}}=0.806 \end{gathered}[/tex]The moles of Cu in 51.226g are 0.806 mol
The molecule below contains both an aldehyde and a ketone functional group. Circle only the carbonyl carbon atom of the aldehyde functional group.
Answers
Let's look at the aldehyde. We must look for a similar structure for your task.
Circle the carbon on the right.
Can you help me with questions Determine the name for the structure given
Answers
Answer: the structure given can be named as 2,3-dichloro-5,6-dimethyl-heptan-4-ol
Explanation:
The question requires us to determine the correct name for the chemical structure given:
To clarify the structure given, let's identify the carbons in the main chain:
Also, we need to identify the functional groups bonded to the carbons in the main chain:
Note that there are three types of functional groups (marked in different colors): 2 Cl atoms (green), 1 hydroxyl group (-OH, blue) and 2 methyl groups (-CH3, purple). Also, note that there are only two methyl groups in the chain, as the carbon numbered as "1" is part of the main chain and not a functional group bonded to it.
Now that we know the size of the main chain, its type (only single bonds between carbons) and the functional groups bonded to it, we can determine the name of the compound:
- main chain of 7 carbons only with single bonds: heptane
- name and position of functional groups: 2,3-dichloro; 4-alcohol, 5,6-dimethyl
Therefore, the complete name of this compound would be:
2,3-dichloro-5,6-dimethyl-heptan-4-ol
Given the following unbalanced equation: K+Cl2 -> KClHow many moles of KCl are produced from 2.50 moles K?
Answers
1) Balance the chemical equation
[tex]2K+Cl_2\rightarrow2KCl[/tex]2) Moles of KCl produced from 2.50 mol K.
The molar ratio between KCl and K is 2 mol KCl: 2 mol K.
[tex]_{}molKCl=2.50molK\cdot\frac{2molKCl_{}}{2molK_{}}_{}=2.50molKCl_{}[/tex]2.50 mol K will poroduce 2.50 mol KCl.
.
Use the equation to complete the activity.
238 234
U ------> TH + a
92 90
The nuclear equation shows the transmutation of a form of Uranium into Thorium and an alpha particle. In one to two sentences, explain whether or not the reaction is balanced.
Answers
The reaction is balanced because the number of atoms of each element or particle is equal on both sides of the equation.
What is a nuclear reaction?Nuclear reaction is the process such as the fission of an atomic nucleus, or the fusion of one or more atomic nuclei and/or subatomic particles in which the number of protons and/or neutrons in a nucleus changes.
The reaction products in a nuclear reaction may contain a different element or a different isotope of the same element.
According to this question, the following fission reaction of uranium is given below:
238/92 → 234/90Th + 4/2α
Based on the above reaction, the sum of the atomic mass and atomic number of the products is 238 and 92 respectively. This means that the equation is balanced.
Learn more about nuclear reactions at: https://brainly.com/question/13315150
#SPJ1
Why wouldn’t an electrolyte conduct electricity in the solid form?
Answers
Answer
When the electrolyte is in solid state, electrons are tightly bound to the atoms and are not easily available to flow. So, they do not conduct electricity well.
Convert 1.36 atm to kPa.1.03 x 103kPa1.80 x 10-3kPa1.38 X 102 kPa1.34 x 10-2kPa
Answers
ANSWER
[tex]\text{ 1.38 }\times\text{ 10}^2\text{ Kpa}[/tex]EXPLANATION
Follow the steps below to convert 1.36atm to Kpa
Recall, that 1 atm is equivalent to 101.325Kpa
[tex]\begin{gathered} \text{ 1 atm }\rightarrow\text{ 101.325Kpa} \\ \text{ 1.36 atm }\rightarrow\text{ x Kpa} \\ \text{ cross multiply} \\ \text{ 1 atm }\times\text{ xKp = 101.325Kpa }\times\text{ 1.36atm} \\ \text{ Isolate xKpa} \\ \text{ xKpa = }\frac{\text{ 101.325}\times\text{ 1.36}}{1} \\ \text{ xKpa = 137.802 Kpa} \\ xKpa\text{ }\approx\text{ 1.38 }\times\text{ 10}^2\text{ Kpa} \end{gathered}[/tex]Therefore, the correct answer is option C (1.38 x 10^2 Kpa)
There are four sketches below. The first sketch shows a sample of Substance X. The three sketches underneath it show three different changes to the sample.
You must decide whether each of these changes is possible. If a change is possible, you must also decide whether it is a physical change or a chemical change.
Each sketch is drawn as if the sample were under a microscope so powerful that individual atoms could be seen. Also, you should assume that you can see the
entire sample, and that the sample is in a sealed box, so that no matter can enter or leave.
Sample of Substance X
oli
Change 1
Change 2
Change 3
Change 1 is:
Impossible
a physical change
Change 2 is:
Impossible
a physical change
Change 3 is
Impossible
a physical change
Explanation
Check
Answers
Physical change: a new substance is not produced.
Chemical change: produces a new substance.
The substance x has one white dot, two black dots and one white again.
W - B - B - W.
If we look at the change 1 we can see that we have one green, two black and another green.
Is it possible to have one green atom? What does that mean? It means that we have another atom there. The problem says that the sample is in a sealed box, so that no matter can enter or leave. So in my opinion the first one is impossible.
In the second change I can't decide between physical and chemical. I think it is a chemical change because there is a rearrengement.
In the third change there are 12 molecules, Each molecule has two black atoms and 4 white atoms. In the substance x only had 24 white atoms, but in the change 3 we have 48 white atoms. As we said before the sample is in a sealed box, so that no matter can enter or leave. So it also impossible.
In lecture, the professor names a molecule 4-ethylpentane. An alert student pointed out that although the correct structure could be drawn, the name did not follow systematic rules. What is the correct systematic name for the molecule?
Answers
Answer
C. 3-methylhexane.
Explanation
In a systematic system of naming organic molecules, te longest chain wof carbon ill be considrered first.
So the longest chain of carbon in the molecule will be 6 (hexane) and not 5 (pentane).
The parent name of the molecule will be hexane.
The only methyl group left is bonded to carbon 3 (numbering in such a way the methyl group s given the least number possible).
Therefore, the correct name of the molecule is 3-methylhexane.
The answer is C. 3-methylhexane.
Use the balanced equation to solve the problem.C3H8 + 502 3CO₂ + 4H₂O-4.16L O₂ gas react with C3Hg at STP.How many grams of H₂O are made?G
Answers
26.67 grams of H₂O are made.
Standard sets of circumstances for experimental measurements are established at standard pressures and temperatures to enable comparisons between various sets of data.
A chemical equation that is balanced has equal amounts of each element's atoms on both sides of the equation and conserves mass.
1 mole of any gas = 22.4 L
We have,
4.16 L of O₂
Now,
The molar mass of H₂O = 18.0 g
By using stoichiometry,
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
We can see that the reaction is completed and balanced.
Therefore,
5 × 22.4L O₂ = 4 × 18.0 g H₂O
Let x be the number of grams of H₂O made after the reaction.
4.16 L O2
x = 4.16 L O₂ × 4 × 18.0 g H₂O / 5 × 22.4 L O₂
x = 2.67 g H₂O
Learn more about grams here:
https://brainly.com/question/15900508
#SPJ9
what would the product be? for A and B?Item A.
Answers
Assuming the reactions are for complete combustion, the products are always carbon dioxide and water, so the unbalanced equation is:
[tex]C_5H_{12}(g)+8O_2\to CO_2(g)+H_2O(g)[/tex]Now, we need to balance it.
As we can see, the carbon atom, C, only appears in one place on both sides, so let's start by balancing it.
We have 5 carbon on the left side, and one carbon of the right side, so we can balance the carbon atom by adding a coefficient of 5 on CO₂:
[tex]C_5H_{12}(g)+8O_2(g)\to5CO_2(g)+H_2O(g)[/tex]Now, we can balance the hydrogen atom, H. We have 12 hydrogen on the left side and 2 per water molecule on the right side. So, we can put the coefficient 6 on the water, so we balance the H atom:
[tex]C_5H_{12}(g)+8O_2(g)\to5CO_2(g)+6H_2O(g)[/tex]Now, we need to check wheter the oxygen atom, O, is balanced.
On the left side, we have 8 times 2, so 16 oxygen.
On the right side we have 5 times 2 on CO₂, so 10, plus 6 on the water, so a total of 16 oxygen.
Since both side are equal, the equation is balanced.
So, the final equation is:
[tex]C_5H_{12}(g)+8O_2(g)\to5CO_2(g)+6H_2O(g)[/tex]body that orbits a planet
Answers
A body that orbits a planet is satellite.
Answer:
A satellite is a body that orbits a planet. the earth natural satellite is the moon. while the body that revolve round the sun is called planets
Explanation:
rate as brainliest
How many grams of NaOH are needed to make 100 ml of a 1 M solution? (numbers only, to the nearest 0.1)
Answers
ANSWER
The mass of NaOH in grams is 4.0 grams
EXPLANATION
Given data
The volume of the solution = 100 ml
The molarity of the solution = 1 M
To find the mass of NaOH in grams. follow the steps below
Step 1: Find the number of moles by applying the molarity formula
[tex]n\text{ = C}\times v[/tex]Where
n = the number of moles
C = Concentration in molarity
v = volume of the solution in liters
Step 2:convert the volume of the solution from ml to l
[tex]\begin{gathered} \text{ 1ml is equivaent to 0.001l} \\ Hence,\text{ } \\ 100ml\text{ = 100 }\times\text{ 0.001} \\ 100ml\text{ = 0.1l} \end{gathered}[/tex]Step 3: substitute the given data into the formula in step 1
[tex]\begin{gathered} n\text{ = cv} \\ n\text{ = 1 }\times\text{ 0.1} \\ n\text{ = 0.1 mol} \end{gathered}[/tex]Hence, the number of moles of NaOH is 0.1 mol
Step 4: Find the mass in grams by using the below formula
[tex]\text{ Mole = }\frac{\text{ mass}}{\text{ molar mass}}[/tex]Recall, that the molar mass of NaOH is 39.997 g/mol
Step 5: substitute the given data into the formula in step 4
[tex]\begin{gathered} \text{ Mole = }\frac{\text{ mass}}{\text{ molar mass}} \\ 0.1\text{ = }\frac{mass}{39.997} \\ cross\text{ multiply} \\ mass\text{ = 0.1 }\times\text{ 39.997} \\ \text{ Mass = 3.997 grams} \\ \text{ Mass}\approx\text{ 4.0 grams} \end{gathered}[/tex]Hence, the mass of NaOH in grams is 4.0 grams