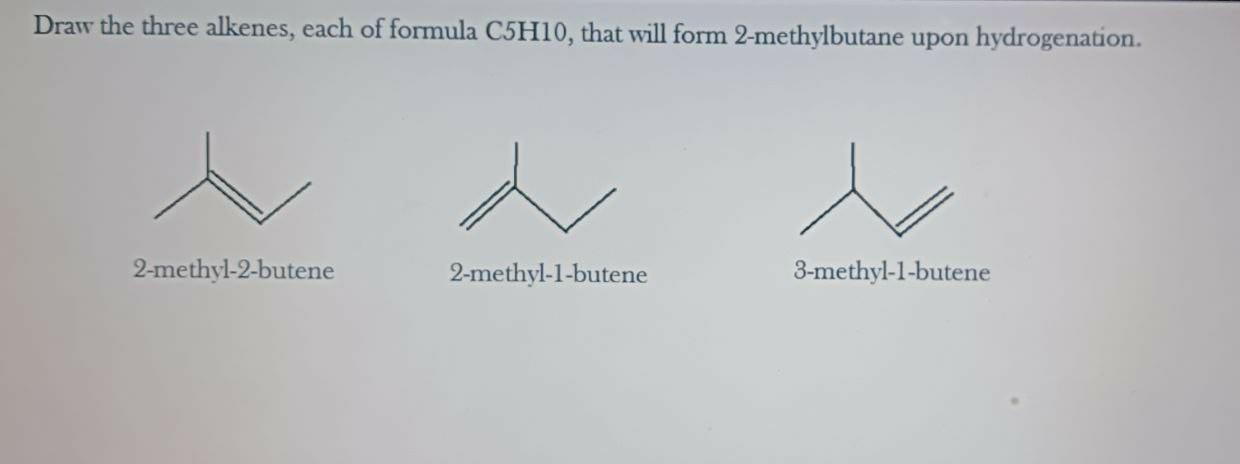
draw the three alkenes, each of formula c5h10 , that will form 2‑methylbutane upon hydrogenation.
Answers
The three alkenes, each of formula C5H10, that will form 2-methylbutane upon hydrogenation will be 2-methyl-2-butene, 2-methyl-1-butene, and 3-methyl-1-butene.
Alkenes are acyclic (branched or unbranched) hydrocarbons having one carbon-to-carbon double bond. The addition of hydrogen to a carbon-carbon double bond is called hydrogenation. The general outcome of such an addition is the reductive reduction of the double-bond functional group. The easiest source of two hydrogen atoms is molecular hydrogen, but integrating alkenes with hydrogen does not result in any noticeable reaction.
Hydrogenation plays a a pivotal role in chemical synthesis. Although the widespread hydrogenation reaction is exothermic, a high activation energy precludes it from taking place under regular conditions.
Learn more about hydrogenation here:
https://brainly.com/question/9810695
#SPJ4
You can find the figure of three alkenes in the attached file.
Related Questions
a 3.5kg object is accelerating at 2.2m/ while being pushed by a force of 10 n to the right across the floor
Answers
The next external force exerted on the papaya is 7.7 N.
What is force?Force is the power that changes the motion of the object. The external force that accelerated the papaya across the table must be resolved in this problem. We can solve the external force by applying the idea of Newton's first law of motion.
F = ma
where
F is the force, the unit is in Newtons (N)
m is the mass, the unit is in kg
a is the acceleration, the unit is in m/s²
Given that, m = 3.5 kg
a = 2.2 m/s²
F = ?
F = ma
Substitute the given data
F = (3.5 kg) (2.2 m/s²)
F = 7.7 N
Therefore, the external force of the papaya is 7.7 N.
To learn more about force, refer to the link:
https://brainly.com/question/26262858
#SPJ1
The question is incomplete. Your most probably complete question is given below:
What is the next external force exerted of the papaya?
A sample of gas weighs 3.33 g and occupies a volume of 1.365 l at 95 °c and 790 torr. identify the gas sample.
a. Cl2 (molar mass 70.90 g/mol)
b. NH3 (molar mass 17.03 g/mol)
c. N2O (molar mass 44.02 g/mol)
d. CHCls (molar mass 119.4 g/mol)
e. SO2 (molar mass 64.07 g/mol)
Answers
The gas sample is of chlorine gas.
The weight of the gas sample is 3.33 grams and it occupies a volume of 1.365 l at 95 degrees Celsius. The pressure is 790 torr.
The ideal gas equation is,
PV = (W/M)RT
Where,
P is the pressure of the gas,
V is the volume of the gas,
W is the weight of the gas
M is the molar mass of the gas,
R is the gas constant,
T is the temperature of the gas.
Putting values,
790 x 1.365 = (3.33/M)62.36 x 368.15
Solving to get the value of M,
M = 70.89 g/mol.
This is near to the mass of the gas Cl₂.
So, the gas is chlorine.
To know more about ideal gas equation, visit,
https://brainly.com/question/27870704
#SPJ4
What does half-life of 4 hours mean?
Answers
The duration needed for a drug's active ingredient to halve in your body is known as the half-life.
The duration needed for a drug's active ingredient to halve in your body is known as the half-life. According to how the body breaks down and eliminates the substance, this will happen. It can range from a few of hours to a couple of days, or even a couple of weeks. It may be possible for you to switch to a similar drug with a longer half-life if you are on a short-half-life medication and experiencing withdrawal symptoms. This medication with a longer half-life might be simpler to stop taking.
The duration of a drug's half-life can also serve as a reference for how long it will take for your body to adjust to its first dose and reach a steady level. It will typically take around.
Learn more about half-life here:
https://brainly.com/question/24710827
#SPJ4
explain why real molecules like water and ammonia have bond angles of 104.5o and 107o instead of 109.5o.
Answers
Answer:
This is because of the lone pairs present in the molecules. Bond and lone pairs of electrons have maximum mutual repulsion but lone pairs of electrons have a stronger mutual repulsion than bond pairs thus creating a bent or v shape in water and trigonal pyramidal shape in ammonia.
There are 4 pairs of electrons in water. if all were bond pairs then the bond angle would be 109.5 but because of the the two lone pairs you take away 2.5 per lone pair from 109.5. Same goes to ammonia if they were all bond pairs then it would be 109.5 but because of the one lone pair it becomes 107.
Explanation:
what is the order of decreasing atomic radius? 1. chlorine, silicon, sulfur 2. silicone, sulfur, chlorine 3. chlorine, sulfur, silicon 4. sulfur, silicon, chlorine 5. sulfur, chlorine, silicon 6. silicon, chlorine, sulfur
Answers
As atomic length decreases on transferring alongside a period, the atom with biggest atomic radius is silicon, then sulfur observed with the aid of using chlorine which has the smallest atomic radius.
The periodic table, atomic radii lower from left to proper throughout a row and boom from pinnacle to backside down a column. Because of those trends, the most important atoms are determined withinside the decrease left nook of the periodic table, and the smallest are determined withinside the higher proper nook (They are in Group 16, 17, and 14 respectively.
As atomic length decreases on transferring alongside a period, the atom with biggest atomic radius is silicon, then sulfur observed with the aid of using chlorine which has the smallest atomic radius.
Read more about atom:
https://brainly.com/question/621740
#SPJ4
how many electrons are in a antibonding molecular orbitals based on the molecular orbital diagram for o2 given that each o electron configuration is 2s22p4 do include electrons from the 1s shell in your count
Answers
Oxygen has an electron configuration of 1s22s22p4. In order to accommodate the twelve valence electrons in O2, molecular orbitals must be large enough. two electrons in orbitals that are antibonded.
Due to less electron-electron repulsion (Hund's Rule), each of these electrons is in a different orbital. The Molecular Orbital theory encompasses the idea of bonding and antibonding electrons, thus we will first discuss the theory. Molecular oxygen has a bond energy of 498 kJ/mole. Given that oxygen possesses two electrons in an antibonding orbital as opposed to nitrogen's single electron, it is not unexpected that this is less than the 945 kJ bond energy of N2. The dioxygen molecule's two unpaired electrons give this material a peculiar and defining characteristic: O2 is paramagnetic.
To learn more about molecular orbital click here https://brainly.com/question/29642622
#SPJ4
a. your instructor had transposed two numbers when reading the pressure and reported the barometric pressure was 745 torr when in fact the correct room pressure was 754 torr?
Answers
In this case mass of the pressure used for the calculation (745 torr) is less than the correct room temperature (754 torr).
What is barometric pressure ?
The measurement of atmospheric pressure, more particularly the weight of air molecules at a given location on Earth.
What is pressure ?
Divided by the surface area of the wall, the total force of all gas particle/wall collisions: One of the basic measurably present quantities of this phase of matter, pressure, is an effect that all gases have.
In this case, mass of the pressure used for the calculation (745 torr) is less than the correct room temperature (754 torr). This will lead to lower calculation of mass being present for Mg metal, since the pressure is 9 torr less than the actual pressure.
Therefore, in this case mass of the pressure used for the calculation (745 torr) is less than the correct room temperature (754 torr).
Learn more about barometric pressure from the link.
https://brainly.com/question/929992
#SPJ4
Using standard heats of formation, calculate the standard enthalpy change for the following reactions.
a. Fe3O4(s)+4H2(g)→3Fe(s)+4H2O(g)
b. C(s,graphite)+O2(g)→CO2(g)
c. NH2Cl(aq)→NH3(g)+HCl(aq)
Answers
The reaction between CH4(g) + 2O2(g) and CO2(g) + 2H2O(l) results in an enthalpy change of -891 kJ/mol.
When given the temperatures of formation, how can we determine the standard enthalpy change?The standard enthalpy change of formation, in this equation, is defined as the product of the standard enthalpies of formation of the reactants and the sum of the standard enthalpies of formation of the products. Along with the data for the normal formation enthalpy: H fo[A]=433 KJ/mol. H fo[B] = -256 KJ/mol. The reaction enthalpy can be calculated by subtracting the sum of the enthalpies of all the reactants from the sum of the enthalpies of the products. By subtracting the sum of the enthalpies of the reactants from the total enthalpies of the product, we may get the thermodynamic constant, or tH.To learn more about Enthalpy change refer to:
https://brainly.com/question/16387742
#SPJ4
Is argon a gas at room temperature and pressure?
Answers
The noble gases of Group 8A argon a gas at room temperature and pressure at room temperature (as the name of the group implies); since they are all unreactive, monatomic elements, their boiling points are extremely low.
The noble gases are, in decreasing order of mass, helium, argon, krypton, xenon, and radon. Since they are so magnificent and don't generally react with anything, they are known as noble gases. Because of this, they are also referred to as inert gases. Noble gases are distinct because, at normal temperature, they are gaseous. The least reactive element, they. Most of the time, they do not mix with other atoms. The noble gases are a group of chemical elements that share many characteristics. They are all monatomic, odourless, colourless gases with very little chemical reactivity under normal conditions. Historically, the term "noble gases" also applied to inert gases.
Learn more about noble gases here:
https://brainly.com/question/11764545
#SPJ4
What is the combining ratio of NaOH and H2SO4?
Answers
Combining ratio of NaOH and H2SO4 is 2:1
The balanced chemical equation is ;
2NaOH + H2SO4 → Na2SO4 + 2H2O.
Combining ratio is the ratio between moles of reactant required to form products it means how many moles of reactant is required to form product in chemical equation.
Here two moles of sodium hidroxide is required to react with one mole of sulphuric acid to form one moles of sodium sulphate and two moles of water.
So, combining ratio of reactant will be 2:1 and ratio fow product will be 1:2
Hence combining ratio of NaOH and H2SO4 is 2:1
To know more about Balance Equation visit here ; https://brainly.com/question/28294176?referrer=searchResults
#SPJ4
50 cm3 of a gas were collected during a reaction. The reaction was carried out at room temperature and pressure. How many moles of the gas were collected? give your answer to 3 decimal places.
Answers
The moles of gas collected during the reaction carried out under normal conditions of pressure and temperature and occupying a volume of 50 cubic centimeters were 0.002 moles.
Calculation of moles of gasTo calculate the number of moles of gas that were collected, the ideal gas formula PV = nrt is used
Procedure to calculate the number of moles of gasAssuming the normal conditions of temperature and pressure, we have the following data
n = ?
V = 50 cm3 = 0.05 L
R = 0.082 L atm / mol °K
t = 25°C = 298°K
P = 1 atm
n = PV / Rt
n = 1 x 0.05 / 0.082 x 298
n = 0.05 / 24,436
n = 0.002 mole
Learn more about moles of gas collected during reactions at https://brainly.com/question/1217205
#SPJ4
gaseous methane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 13. g of methane is mixed with 99.0 g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. be sure your answer has the correct number of significant digits.
Answers
The mass of the carbon dioxide that could be produced by the chemical reaction is 11.68 gram.
In Any chemical reactions involving multiple reactant species one of the reactants present has an initial molar quantity that does not satisfy the necessary stoichiometry of the balanced reaction equation. If the reaction proceeds to completion, then this limiting reactant is fully consumed.
The balanced complete combustion reaction equation of methane is,
CH4 + 2 O2 ---> CO2 + 2H20
Mass of methane reactant = 13g
Moles of methane reactant = 13g * 1 mole/ 16.0 gram = 0.81 mole
Mass of oxygen reactant = 99g
Moles of oxygen reactant = 99g * 1 mole/ 32.0gram = 3.093 mole
According to the balanced equation oxygen is the limiting reactant. Therefore methane is the excess reactant. Some of its quantity remains after complete reaction.
Moles of methane reactant remaining= 0.81 mole - 3.093/2 mole
= 0.73 mole
Mass of methane reactant remaining = 0.73 mole * 16.0gram /1 mole
= 11.68 gram
To learn more about Mass calculation please visit
https://brainly.com/question/837939
#SPJ4
term a client with a serum sodium level of 125 meq/ml should benefit most from the administration of which intravenous solution? a. 0.9% sodium chloride solution (normal saline) b. 0.45% sodium chloride solution (half normal saline) c. 10% dextrose in 0.45% sodium chloride d. 5% dextrose in 0.2% sodium chloride
Answers
The administration of 0.9% sodium chloride solution will be most beneficial for a patient with a blood sodium level of 125 meq/ml (normal saline).
The patient has hyponatremic hyponatraemia (normal range for serum sodium is 135–145 mEq/mL or mmol/L). In comparison to 0.45% sodium chloride solution (half normal saline), 10% dextrose in 0.45% sodium chloride, and 5% dextrose in 0.2% sodium chloride, 0.9% sodium chloride solution or normal saline offers the most sodium. All hypotonic fluids contain sodium chloride, sometimes known as saline. The sodium chlorine content of hypotonic fluids is lower than that of isotonic and hypertonic IVs. In light of this, these solutions are applied to patients who exhibit hypernatremia or excessive sodium chloride levels. Patients with fluid volume deficiency are treated with isotonic solutions (also known as hypovolemia) to raise their blood pressure.
To learn more about normal saline click here https://brainly.com/question/4760027
#SPJ4
the chelate effect describes the increased affinity for a polydentate ligand versus a ligand of lower denticity. consider the reaction considering the bonds formed and bonds broken in the reaction, what is the sign of enthalpy change in this reaction?
Answers
Entropy (+ S) increases noticeably when monodentate ligands are replaced by chelating ligands. This is what the Chelate Effect is mostly caused by.
To give one example, the total number of molecules rises from two to three when en takes the place of two ammonia ligands .Just one more molecule is all that is required to accelerate the reaction.
In the aforementioned example, there is only one bidentate ligand in play. The chelate action is further amplified when numerous bidentate ligands are involved or when denticity rises. Think about the two complexation equilibria between ammonia, NH3, and the cobalt (II) ion, Co2+(aq), ethylenediamine (en), on the one hand, and the cobalt (II) ion, Co2+(aq) and en.
the entropy caused by an increase or decrease in the number of freely moving molecules. The number of free molecules in the system rises when a chelating ligand takes the place of multiple monodentate ligands, leading to a sizable increase in entropy. The chelate effect is primarily driven by this energy component.
To know more about chelate effect, please refer:
https://brainly.com/question/15242975
#SPJ4
what relative masses of dimethyl amine and dimethyl ammonium chloride do you need to prepare a buffer solution of ph
Answers
For dimethylamine:
Acidity (pKa) 10.64
Basicity (pKb) 3.36
What is Buffer Solution?
The buffer solution, a water-based solvent solution, is created by combining a weak acid and its conjugate base, or a weak base and its conjugate acid. They can be diluted or have small amounts of acid or alkali added to them without the pH of the solution altering.
The pH of buffer solutions scarcely changes when a modest quantity of a strong acid or strong base is introduced. They are therefore used to keep the pH level constant.
Consider this for a moment: If the molar ratio of dimethylamine to dimethylammonium chloride were 1:1, pH would be equal to pKa. At that point, you would be at the half-equivalence point. 10.64 would be the pH.
However, you need a buffer whose pH is 10.43. A molar excess of dimethylammonium chloride must thus exist.
Applying the H-H equation
pH equals pKa plus log ([[base]/[salt]])
10.43 = 10.64 + log ([base[/salt]) log ([base[/salt]) = 10-43 - 10.64 log ([base]/[salt]) = -0.21 log ([base]/[salt]) = 10-0.21 log ([base]/[salt]) = 0.62
The buffer has to be made by combining 1.0mol of dimethylammonium chloride and 0.62mol of dimethylamine.
Mass to volume as necessary:
Dimethylammonium chloride, molar mass 81.55g/mol
Molar mass of dimethylamine is equal to 45.08g/mol minus 0.62mol, or 27.95g.
Dimethylamine to dimethylammonium chloride mass ratio is 81.55 to 27.95.
OR Ratio is 2.92:1.0 (81.55/27.95).
Learn more about Buffer Solution from given link
https://brainly.com/question/26416276
#SPJ4
Why does atomic radius increase across Period 3?
Answers
An atomic radius decrease across Period 3 because the number of shells not increases but the number of electron increases and the atomic size decreases as the effective nuclear charge increases.
When we move in the period 3 from left to the right the atomic radius decreases because of the reason that the the number of shells don not change they will remain the same across the period but the number of the electron will increases . the effective nuclear charge will also increases. the nucleus will attract the electrons more strongly that the reason the atomic radius decreases.
Thus, number of electron increases and the number of shells will remain same , the atomic size decreases as the effective nuclear charge increases.
To learn more about atomic radius here
https://brainly.com/question/6814005
#SPJ4
Minerals or organic matter carried and deposited by water or wind that may consolidate into rock.
a. True
b. False
Answers
It is true that minerals or biological stuff can be transported by the wind or water, where they are then deposited and may solidify into rock.
What minerals exist in organic material?There are also elemental carbon minerals like graphite and diamond, as well as carbides, simple carbon oxides like carbon monoxide and carbon dioxide, carbonates, cyanides, and other carbon compounds. Often forming crusts over cracks, organic minerals are uncommon and difficult to locate. Mineral, biological, or pre-existing rock grains that can be carried by water, wind, or ice are classified as sediment. Sedimentary rock is defined as rock that forms at or very close to the Earth's surface as a result of the consolidation of loose sediment that has gathered due to wind, water, or glacier deposition.To learn more about Mineral refer to:
https://brainly.com/question/15844293
#SPJ4
why did the big bang not produce a significant proportion of elements heavier than helium?
Answers
Helium could only persist until the temperature dropped too low for heavier elements to develop.
Why did the Big Bang not yield a lot of heavy elements?Due to a bottleneck caused by the lack of a stable nucleus with 8 or 5 nucleons, Big Bang nucleosynthesis created extremely few nuclei of elements heavier than lithium. Additionally, the amount of lithium-7 created during BBN was constrained by this deficiency of bigger atoms.
Why did not heavier atoms than helium emerge during the first few seconds of creation?Before few minutes into the expansion, the early cosmos was too hot for atoms to form. There were only a few minutes left before it was too cold to continue nucleosynthesis and no more elements could start to form.
To know more about helium visit:-
https://brainly.com/question/1780577
#SPJ4
Malonyl-CoA is an intermediate in fatty acid synthesis. It also regulates fatty acid metabolism. Which of the following molecules regulate the enzyme that catalyzes malonyl-CoA synthesis? a. fatty acids or fatty acyl-CoA b. carnitine c. oxaloacetate d. acetyl-CoA e. citrate Which of the following enzymes does malonyl-CoA regulate? a. ATP-citrate lyase b. carnitine acyltransferase c. acetyl-CoA carboxylase
d. fatty acid synthase e. acyl-CoA dehydrogenase
Answers
Malonyl-CoA is an important intermediate in fatty acid synthesis and is essential for regulating fatty acid metabolism. It is synthesized from acetyl-CoA, which is the end-product of the citric acid cycle and a major energy source for cells.
Which of the following molecules regulate the enzyme that catalyzes malonyl-CoA synthesis? Option D. Acetyl-CoAAcetyl-CoA is the molecule that regulates the enzyme that catalyzes malonyl-CoA synthesis. Acetyl-CoA is the end-product of the citric acid cycle and is a major source of energy for cells. It is also the starting point for fatty acid biosynthesis. Acetyl-CoA is used to synthesize malonyl-CoA, which is then used in the elongation of fatty acids. Acetyl-CoA is the molecule that activates the enzyme involved in fatty acid synthesis, acetyl-CoA carboxylase, which is responsible for the conversion of acetyl-CoA to malonyl-CoA. Therefore, acetyl-CoA is the molecule that regulates the enzyme that catalyzes malonyl-CoA synthesis.
Which of the following enzymes does malonyl-CoA regulate? Option C. Acetyl-CoA carboxylaseMalonyl-CoA regulates the enzyme acetyl-CoA carboxylase, which is responsible for the conversion of acetyl-CoA to malonyl-CoA. Acetyl-CoA carboxylase is an enzyme that catalyzes the formation of malonyl-CoA from acetyl-CoA, which is the starting point for fatty acid synthesis. Malonyl-CoA is then used in the elongation of fatty acids. Therefore, acetyl-CoA carboxylase is the enzyme that malonyl-CoA regulates.
Learn more about Enzymes: https://brainly.com/question/1596855
#SPJ4
How many total electrons must be transferred to form one formula unit of the compound al2o3?.
Answers
Six electrons are transferred in the formation of Al₂O₃.
Aluminum metal and Oxygen react to form Al₂O₃ as,
2 Al + 3/2 O₂ → Al₂O₃
Oxidation number of Al on left hand side is zero, while than on right hand side in Al₂O₃ is +3.
It means it has lost 3 electrons per one atom and six electrons per two atoms. Also, the oxidation number of O at left hand side in O₂ is zero, while that in Al₂O₃ it is -2 per atom and -6 per 3 atoms.
So, two Al atoms have lost 6 electrons and 3 O atoms have gained six electrons.
To look more about formula unit click here
brainly.com/question/21494857
#SPJ4
Answer:
6
Explanation:
Al has a left-side oxidation number of zero and a right-side oxidation number of three. means that it has lost three electrons for every single atom and six for every two atoms. Additionally, the left-hand side of O2 has an oxidation number of zero, whereas Al2O3 has an oxidation number of -2 per atom and -6 per three atoms. Thus, three O atoms have received six electrons whereas two Al atoms have lost 6 electrons.
the gas in a 250.0 ml piston experiences a change in pressure from 1.00 atm to 3.45 atm. what is the new volume (in ml) assuming the moles of gas and temperature are held constant?
Answers
The new volume (in ml) assuming the moles of gas and temperature are held constant is 72.4 ml.
What is pressure?
A barometer or manometer can be used to measure pressure, which is the force applied per unit area. For an accurate physical description of a sample of a gas, four variables must be known: temperature, volume, quantity, and pressure. The SI unit for pressure is the pascal (Pa), which equals one newton per square meter (N/m²) of surface area. Pressure is defined as force per unit area of surface. An object's pressure is inversely proportional to the area on which the force is applied, and it is proportional to the force it exerts.
We have,
At constant temperature = P₁V₁ = P₂V₂
Where,
Initial pressure, P₁ = 1.00 atm
Final pressure, P₂ = 3.45 atm
Initial volume, V₁ = 250 ml
Final volume, V₂ = ?
Putting values in the formula
=(1.00 atm) × (250ml) = (3.45 atm) × V₂
V₂ = 72.4 ml
To know more about pressure, check out https://brainly.com/question/28012687
#SPJ4
which of the following correctly describes an ideal gas? multiple choice question. a gas that obeys the gas laws under all conditions a gas that suits a particular application perfectly a gas that is classified as a noble gas a gas that flows like a liquid
Answers
Among the options that correctly describes an ideal gas is a gas that obeys the gas laws under all conditions.
Ideal gas, also known as perfect gas, is a gas that physically complies with the ideal, or general, gas law, a particular idealised relationship between pressure, volume, and temperature. This law states that for a given amount of gas, the sum of the volume V and pressure P is proportional to the absolute temperature T; in equation form, PV = kT, where k is a constant. It is a generalisation that includes both Boyle's law and Charles's law as special cases. Such a relationship is known as a substance's equation of state, and it is sufficient to explain its basic behaviour. The kinetic theory of gases, which makes the assumptions, can be used to derive the ideal gas law.
To know more about ideal gas visit : https://brainly.com/question/12281987
#SPJ4
What happens at the transition stage?
Answers
At the transition stage, one form of matter starts getting converted into another form of matter.
Solid, liquid and gas are the three phases in which matter occur in nature. Matter is interconvertible in nature i.e it can be converted from one form to another.
Melting, freezing and evaporation are the three phases of transition.
Liquid water at low temperature freezes to form solid ice. This process is known as freezing. Whereas, liquid water when provided high temperature or heat releases vapours. This process is known as evaporation. Solid ice when brought at room temperature gets converted back into liquid water. This process is known as melting.
To know more about transition stage here
https://brainly.com/question/19983755
#SPJ4
the mass of a nitrogen molecule is 14 times greater than that of a hydrogen molecule. what is the temperature of a sample of hydrogen whose average molecular energy is equal to that in a sample of nitrogen at 300 k?
Answers
The kinetic strength of one mol of nitrogen molecules at three hundred K is 3741.3 Joules.The KE is 41 kj.
We recognize that,Kinetic strength ∝ TIn the question, temperature is equal in each conditions. So, the kinetic strength withinside the 2d situation is E.Room imply rectangular velocity is given by
vrms=√3RTM
Here,M= Molar mass of fueloline moleculeT= temperature of the fueloline moleculeWe have given VN2=VH2
∴√3RTN2MN2=√3RTH2MH2
⇒TH22=57328⇒TH2≈41 K
Read more about nitrogen:
https://brainly.com/question/1380063
#SPJ4
a given sample of n2 gas has a pressure of 0.30 atm at 30.0 °c. if the volume is 2.0 l, how many moles of n2 are present?
Answers
A given sample of n2 gas has a pressure of 0.30 atm at 30.0 °c. if the volume is 2.0 l, then moles of n2 are present is 0.002.
PV=nRT
n=PV/RT
n=0.30×2÷0.82×298
n=0.6/244
n=0.002 mol
The mole concept is a helpful way to quantify the amount of a substance. When dealing with particles at the atomic (or molecular) level, it is known that even one gramme of a pure element contains an enormous number of atoms. The mole concept is frequently used in this context. The most popular unit of measurement is the "mole," which is a count of a significant number of particles. The number 6.02214076*1023, also known as the Avogadro constant, is frequently denoted by the letter "NA". Among the elementary entities that can be represented in moles are atoms, molecules, monoatomic and polyatomic ions, as well as other particles (such as electrons).
To know more about moles visit : brainly.com/question/15209553
#SPJ4
9. What is the balanced formula equation for the reaction shown in the figure?
a. H2 + Br2 ---> 2HBr
b. 2H + 2Br ---> 2HBr
c. H2 + B2r ---> H2Br2
d. H2 + Br2 ---> HBr + HBr
10. Identify the product(s) in the chemical equation H2 + Br2 ---> HBr
a. hydrogen only
b. both hydrogen and bromine
c. hydrogen and bromine only
d. both bromine and hydrogen bromide​
Answers
in a nuclear fusion reaction, which has more mass: the initial hydrogen isotopes or the fusion products?
Answers
the initial hydrogen isotopes in a nuclear fusion reaction have more mass than the fusion products.
Powering the Sun and other stars are nuclear fusion process. Two light nuclei combine to form one heavy nucleus during a fusion process. Because the mass of the single nucleus formed is less than the combined mass of the two initial nuclei, energy is released throughout the process. Energy is created from the remaining mass. The reason this happens is explained by Einstein's equation (E=mc2), which says that mass and energy can be transformed into one another. Fusion energy could play a significant role in the creation of energy if scientists can figure out how to use it in Earth-based machinery. Numerous elements from the periodic table can undergo fusion. However, the deuterium-tritium (DT) fusion reaction is of particular interest to scientists studying fusion energy applications. DT fusion generates a neutron and a helium nucleus.
learn more about nuclear fusion here;
https://brainly.com/question/17870368
#SPJ4
elect True or False for each statement: The solubility of gases in water decreases with increasing temperature Most solids are more soluble at higher temperature. Pressure has little effect on the solubility of liquids and solids because they are almost incompressible.
Answers
True, The solubility of gasses in water decreases with an increase in temperature.
What is solubility?
A substance's solubility is the greatest amount that will dissolve in a given amount of solvent at a given temperature. A specific solute-solvent combination's solubility is a defining trait, and the solubility of various compounds can vary significantly.
When temperature rises, molecules' vibrational kinetic energy increases, breaking intermolecular bonds and allowing gases to escape from the solution, the solubility of gases in water is inversely proportional to temperature.
Hence, the solubility of gasses in water decreases with increase in temperature.
To learn more about solubility from the given link:
https://brainly.com/question/9098308
#SPJ4
A compound is found to have an empirical formula of ch2o. what is the molecular formula when the molecular weight is 180.0 grams?
Answers
The molecular formula is C₂H₄O₂ when molecular weight is 180 g having empirical formula of CH₂O.
The compound's empirical formula is CH₂O, which contains two hydrogen atoms and one oxygen atom for every carbon atom.
CH₂O has a mass of 30 (=12+2*1+16).The molecule has a molecular weight of about 180.As a result, the supplied molecule has the molecular formula C₆H₁₂O₆.
What is the chemical structure of the equation for water, CH₂O?
A substance that is organic and has a molar mass pf 60 grams / mole as well as an empirical formula of CH₂O also has a molecular formula or C₂H₄O₂.
How much CH₂O is in a mole?
A mol of CH₂O has a molar mass of 30.026 g.
The periodic chart must first be used to estimate its atomic mass of the each element in the compound.
Therefore, the molecular formula is C₂H₄O₂ with the empirical formula of CH₂O.
To know more about CH₂O visit: brainly.com/question/5523263
#SPJ4
octahedral complexes has two more unpaired electrons in the high-spin configuration than in the low-spin configuration?
Answers
The example of this octahedral complex which has two more unpaired electrons in the high spin configuration than in the low spin configuration is Cobalt (II) or Co2+.
What are Octahedral complexes?
Octahedral complexes are coordination complexes with an octahedral molecular geometry. This geometry is characterized by six ligands attached to a central metal atom at the corners of an octahedron. Octahedral complexes are often used as catalysts in industrial processes, and they are also found in biological systems. The ligands can be either anionic, neutral, or cationic, and the central metal atom can be any of the transition metals from groups 3-12 of the periodic table. Octahedral complexes are often described in terms of their electron configuration, which is determined by the number of electrons in the ligands and the central metal atom. These complexes are important in many areas of chemistry, including inorganic, physical, and organometallic chemistry.
Octahedral complexes contains 6 ligands. The hybridization can be d2sp3 or sp3d2.
There are two types of spin complexes
High spin complexes: When Δo>PLow spin complexes: When Δo< PFor more information refer the attached file below
To know more about Octahedral complexes please visit:
https://brainly.com/question/11856948
#SPJ4