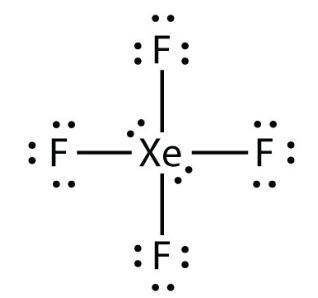
draw the lewis structure for xenon tetrafluoride (xef4). how many valence electrons surround the central xe atom in this structure?
Answers
36 valence electrons surround the xenon atom in XeF4.
What is Lewis structure?
The valence shell electrons in a molecule are represented in an excessively simple form by a Lewis Structure. It is used to illustrate how the electrons in a molecule are placed around specific atoms.
Through using present periodic table, we first count the valence electrons present in the XeF4 molecule. Once they have been determined, we can arrange them around the main atom and attempt to fill each atom's outer shell. Thus, there are 8 + 4 * 7 = 36 valence electrons in XeF4. In order to obtain the optimum Lewis structure for the molecule, formal charge is taken into consideration. Remember that fluorine (F) cannot form a double bond because of its highly electronegative nature, and that xenon (Xe) can contain up to 8 valence electrons.
Therefore, 36 valence electrons surround the xenon atom in XeF4.
To learn more about Lattice energy from the given link.
https://brainly.com/question/20300458
#SPJ4
Related Questions
chemical combination of two or more atoms that form a specific chemical compound
Answers
A molecule is a chemical compound that is created when two or more atoms are combined. Water is an illustration of a molecule (H2O H 2 O ).
Which of the following pairs of atoms, when combined chemically, results in a particular chemical compound?A structure made up of two or more atoms that are chemically connected to one another is called a molecule. A compound or elemental molecule could be the structure.
What is a substance created chemically when two or more elements are combined in specific ratios?Two or more elements are mixed in specific amounts and precise proportions through chemical bonding to form compounds. A substance is a pure form of matter that cannot be physically separated from or refined further.
To know more about chemical combination visit:-
https://brainly.com/question/747874
#SPJ4
Question:-
Which of the following is a chemical combination of two or more atoms that form specific chemical compound?
a sample of iodine-131 has an activity of 200 mci. if the half-life of iodine-131 is 8.0 days, what activity is observed after 16 days?
Answers
The half-life of iodine-131 is 8.0 days
Activity= 200mci
After 16 days the activity would be= 100mci
What is iodine?
Iodine happens in many oxidation states, along with iodide (I−), iodate and the various periodate anions. it's far the least considerable of the stable halogens, being the sixty-first maximum considerable detail. Because the heaviest vital mineral nutrient, iodine is needed for the synthesis of thyroid hormones. Iodine deficiency influences approximately billion people and is the leading preventable motive of highbrow disabilities.The dominant producers of iodine nowadays are Chile and Japan. Due to its high atomic wide variety and simplicity of attachment to natural compounds, it has also discovered favor as a non-toxic radiocontrast cloth. Because of the specificity of its uptake by the human frame, radioactive isotopes of iodine can also be used to treat thyroid cancer.To know more about iodine, click the link given below:
https://brainly.com/question/3059732
#SPJ4
calculate the volume of 6.00 m hbr in mililiters that would be required to react with 2.46 g of na2co3
Answers
The volume of 6.00M HBr in milliliters that would be required to react with 2.46 g of na2co3 is 7.6mL
The balanced chemical equation is:
Na2CO3(s)1 mol+2HBr(aq)2 mol→2NaBr(aq)+H2O+CO2
Moles of Na2CO3 present =2.46/106=0.023
(Molar mass =2×23+12+3×16=106)
Now, according to the equation,
1 mol of Na2CO3 requires 2 moles of HBr
0.023 mol of Na2CO3 requires moles of HBr = 2×0.023=0.046 mol
Now, 6 mole of 6M HCl is present in 1000 mL
0.046 mol of 6M HCl would be present in =(1000/6)×0.046 =7.6 mL
What is volume?
Volume is a measure of the occupied three-dimensional space. Often quantified numerically using SI units (such as cubic meters and liters) or using various imperial or US customary units (such as gallons, quarts, and cubic inches).To know more about volume, click the link given below:
https://brainly.com/question/14710169
#SPJ4
Minerals or organic matter carried and deposited by water or wind that may consolidate into rock.
a. True
b. False
Answers
It is true that minerals or biological stuff can be transported by the wind or water, where they are then deposited and may solidify into rock.
What minerals exist in organic material?There are also elemental carbon minerals like graphite and diamond, as well as carbides, simple carbon oxides like carbon monoxide and carbon dioxide, carbonates, cyanides, and other carbon compounds. Often forming crusts over cracks, organic minerals are uncommon and difficult to locate. Mineral, biological, or pre-existing rock grains that can be carried by water, wind, or ice are classified as sediment. Sedimentary rock is defined as rock that forms at or very close to the Earth's surface as a result of the consolidation of loose sediment that has gathered due to wind, water, or glacier deposition.To learn more about Mineral refer to:
https://brainly.com/question/15844293
#SPJ4
100. ml of 0.200 m hcl is titrated with 0.250 m naoh . part a what is the ph of the solution after 50.0 ml of base has been added? express the ph numerically.
Answers
The ph numerically is 7.00.
What is ph?
Measure of an aqueous solution's basicity or acidity in numbers. The phrase, which is frequently used in chemistry, biology, and agronomy, converts the hydrogen ion concentration, which typically ranges between 1 and 1014 gram-equivalents per litre, into numbers between 0 and 14.
What is titration?
A exact amount of a different substance—one that the desired constituent reacts with in a specific, known proportion—is added to the sample under test in the process of titration, a type of chemical analysis, to determine how much of each constituent is present.
moles HCl = 0.100 L x 0.200 M = 0.0200
moles NaOH = 0.0500 L x 0.250 M=0.0125
moles HCl in excess = 0.0200 - 0.0125 =0.00750
total volume = 0.150 L
[H+]= 0.00750/ 0.150L=0.0500 M
pH = - log 0.0500=1.30
at the equivalence point moles H+ = moles OH- so pH = 7.00
Therefore, the ph numerically is 7.00.
Learn more about Ph from the given link.
https://brainly.com/question/26424076
#SPJ4
A sample of gas weighs 3.33 g and occupies a volume of 1.365 l at 95 °c and 790 torr. identify the gas sample.
a. Cl2 (molar mass 70.90 g/mol)
b. NH3 (molar mass 17.03 g/mol)
c. N2O (molar mass 44.02 g/mol)
d. CHCls (molar mass 119.4 g/mol)
e. SO2 (molar mass 64.07 g/mol)
Answers
The gas sample is of chlorine gas.
The weight of the gas sample is 3.33 grams and it occupies a volume of 1.365 l at 95 degrees Celsius. The pressure is 790 torr.
The ideal gas equation is,
PV = (W/M)RT
Where,
P is the pressure of the gas,
V is the volume of the gas,
W is the weight of the gas
M is the molar mass of the gas,
R is the gas constant,
T is the temperature of the gas.
Putting values,
790 x 1.365 = (3.33/M)62.36 x 368.15
Solving to get the value of M,
M = 70.89 g/mol.
This is near to the mass of the gas Cl₂.
So, the gas is chlorine.
To know more about ideal gas equation, visit,
https://brainly.com/question/27870704
#SPJ4
Rank from highest to lowest boiling point. To rank items as equivalent, overlap them.
1)paraffin, C36H74
2)methane, CH4
3)hexane, C6H14
4)2-2dimethylbutane, C6H14
5)Hexadecane, C16H34
Answers
Rank from highest to lowest boiling point
paraffin, C36H74 hexadecane, C16H34hexane, C6H14 2-2dimethylbutane, C6H14methane, CH4 What is boiling point point?The temperature at which a liquid's vapor pressure equals the pressure around it and the liquid transforms into a vapor is known as the boiling point of a substance.It measures the point at which a chemical boils, to put it simply. A higher boiling point denotes more intermolecular forces and, thus, less vapour pressure, similar to a higher melting point.A liquid's boiling point is influenced by temperature, air pressure, and the liquid's vapor pressure. Boiling starts when the liquid's vapor pressure and air pressure are equal.To learn more about boiling point refer,
https://brainly.com/question/12408418
#SPJ4
what is the order of decreasing atomic radius? 1. chlorine, silicon, sulfur 2. silicone, sulfur, chlorine 3. chlorine, sulfur, silicon 4. sulfur, silicon, chlorine 5. sulfur, chlorine, silicon 6. silicon, chlorine, sulfur
Answers
As atomic length decreases on transferring alongside a period, the atom with biggest atomic radius is silicon, then sulfur observed with the aid of using chlorine which has the smallest atomic radius.
The periodic table, atomic radii lower from left to proper throughout a row and boom from pinnacle to backside down a column. Because of those trends, the most important atoms are determined withinside the decrease left nook of the periodic table, and the smallest are determined withinside the higher proper nook (They are in Group 16, 17, and 14 respectively.
As atomic length decreases on transferring alongside a period, the atom with biggest atomic radius is silicon, then sulfur observed with the aid of using chlorine which has the smallest atomic radius.
Read more about atom:
https://brainly.com/question/621740
#SPJ4
Why does atomic radius increase across Period 3?
Answers
An atomic radius decrease across Period 3 because the number of shells not increases but the number of electron increases and the atomic size decreases as the effective nuclear charge increases.
When we move in the period 3 from left to the right the atomic radius decreases because of the reason that the the number of shells don not change they will remain the same across the period but the number of the electron will increases . the effective nuclear charge will also increases. the nucleus will attract the electrons more strongly that the reason the atomic radius decreases.
Thus, number of electron increases and the number of shells will remain same , the atomic size decreases as the effective nuclear charge increases.
To learn more about atomic radius here
https://brainly.com/question/6814005
#SPJ4
a given sample of n2 gas has a pressure of 0.30 atm at 30.0 °c. if the volume is 2.0 l, how many moles of n2 are present?
Answers
A given sample of n2 gas has a pressure of 0.30 atm at 30.0 °c. if the volume is 2.0 l, then moles of n2 are present is 0.002.
PV=nRT
n=PV/RT
n=0.30×2÷0.82×298
n=0.6/244
n=0.002 mol
The mole concept is a helpful way to quantify the amount of a substance. When dealing with particles at the atomic (or molecular) level, it is known that even one gramme of a pure element contains an enormous number of atoms. The mole concept is frequently used in this context. The most popular unit of measurement is the "mole," which is a count of a significant number of particles. The number 6.02214076*1023, also known as the Avogadro constant, is frequently denoted by the letter "NA". Among the elementary entities that can be represented in moles are atoms, molecules, monoatomic and polyatomic ions, as well as other particles (such as electrons).
To know more about moles visit : brainly.com/question/15209553
#SPJ4
computational chemistry calculates physical and chemical properties of compounds based upon
Answers
Computational chemistry uses methods of theoretical chemistry, incorporated into the computer programs, to calculate structures and properties of molecules, groups of molecules, and the solids.
A subfield of chemistry called computational chemistry makes use of computer modeling to help solve chemical issues. It is crucial since the quantum many-body issue cannot be analytically solved, much less in closed form, with the exception of very recent results pertaining to the hydrogen molecular ion. While computational findings typically supplement the knowledge gained from chemical experiments, they can occasionally forecast previously unknown chemical processes. It is widely employed in the development of novel materials and medications.
Structure, or the predicted positions of the constituent atoms, absolute and relative (interaction) energies, electronic charge density distributions, dipoles and higher multipole moments, vibrational frequencies, reactivity, or other spectroscopic quantities, and cross sections for collision with other particles are examples of these properties.
To know more about computational chemistry, please refer:
https://brainly.com/question/21132543
#SPJ4
how many electrons are in a antibonding molecular orbitals based on the molecular orbital diagram for o2 given that each o electron configuration is 2s22p4 do include electrons from the 1s shell in your count
Answers
Oxygen has an electron configuration of 1s22s22p4. In order to accommodate the twelve valence electrons in O2, molecular orbitals must be large enough. two electrons in orbitals that are antibonded.
Due to less electron-electron repulsion (Hund's Rule), each of these electrons is in a different orbital. The Molecular Orbital theory encompasses the idea of bonding and antibonding electrons, thus we will first discuss the theory. Molecular oxygen has a bond energy of 498 kJ/mole. Given that oxygen possesses two electrons in an antibonding orbital as opposed to nitrogen's single electron, it is not unexpected that this is less than the 945 kJ bond energy of N2. The dioxygen molecule's two unpaired electrons give this material a peculiar and defining characteristic: O2 is paramagnetic.
To learn more about molecular orbital click here https://brainly.com/question/29642622
#SPJ4
What does phosphate do to chlorine?
Answers
The efficiency of chlorine is hampered by phosphates, which also indirectly raise chlorine demand.
Because of this, our third pillar of proactive pool maintenance is the removal of phosphates to maintain their levels below 500 ppb (g/L). Because they weren't always a problem, phosphorus in pool chemistry is a misunderstood problem.
Phosphates are chemical substances that are frequently found in water and are formed of phosphorous and oxygen. PO4, also referred to as orthophosphate, is the most prevalent basic phosphate unit. Phosphates can be produced from phosphoric acid as salts or esters, to put it simply. Phosphates are important in swimming pools because they provide food for pollutants like algae to reproduce.
To know more about phosphate, visit;
brainly.com/question/16094787
#SPJ4
What mass of Na2SO4 is produced if 49 gram of H2SO4 reacts with 80 gram of NaOH?
Answers
71 g of Na2SO4 is produced if 49 gram of H2SO4 reacts with 80 gram of NaOH
Given that;
Mass of Na2SO4 is 49 gram
Mass of NaOH is 80 gram
To find number of mol we have to find molar mass of each element ;
Molar mass of Na2SO4 = 98 g/mol
Molar mass of NaOH = 40 g/mol
So, Number of mol(n) of Na2SO4 = 49/98
n(Na2SO4) = 0.5 mol of H2SO4
Number of mol(n) of NaOH = 80/40
n(NaOH) = 2 moles of NaOH
According to the balanced chemical reaction ; H2SO4 + 2 NaOH –––> Na2SO4 + 2 H2O one mol of H2SO4 react with 2 mol of NaOH to form 1 mol of Na2SO4, same as 0.5 mol of H2SO4 react with 1 mol of NaOH to form 0.5 mol Na2SO4
H2SO4 + 2 NaOH –––> Na2SO4 + 2 H2O
(1) .. .. .. .. (2) .. .. .. .. .. .. (1) .. .. Then,
(0.5) .. .. .. (1) .. .. .. .. .. .. (0.5)
H2SO4 is the limiting and NaOH is the excess reagent.
So,0.5 mol of Na2SO4 is formed .
Mass of Na2SO4 = mol of Na2SO4 × molar mass of Na2SO4
Mass of Na2SO4 = 0.5 × 142
Mass of Na2SO4 = 71 g of Na2SO4
So, 71 g of Na2SO4 will produced
To know more about limiting reagent visit here ; https://brainly.com/question/11848702?referrer=searchResults
#SPJ4
9. What is the balanced formula equation for the reaction shown in the figure?
a. H2 + Br2 ---> 2HBr
b. 2H + 2Br ---> 2HBr
c. H2 + B2r ---> H2Br2
d. H2 + Br2 ---> HBr + HBr
10. Identify the product(s) in the chemical equation H2 + Br2 ---> HBr
a. hydrogen only
b. both hydrogen and bromine
c. hydrogen and bromine only
d. both bromine and hydrogen bromide​
Answers
what’s a wave quantity
Answers
Explanation:
The term wave number refers to the number of complete wave cycles of an electromagnetic field (EM field) that exist in one meter (1 m) of linear space. Wave number is expressed in reciprocal meters (m-1). The wave number for an EM field is equal to 2 pi divided by the wavelength in meters.
The frequency ( ) of a wave is the number of waves passing a point in a certain time. We normally use a time of one second, so this gives frequency the unit hertz ( ), since one hertz is equal to one wave per second.
Three quantities used for measuring waves are
wavelength, frequency and amplitude.pls rate as brainliest
50 cm3 of a gas were collected during a reaction. The reaction was carried out at room temperature and pressure. How many moles of the gas were collected? give your answer to 3 decimal places.
Answers
The moles of gas collected during the reaction carried out under normal conditions of pressure and temperature and occupying a volume of 50 cubic centimeters were 0.002 moles.
Calculation of moles of gasTo calculate the number of moles of gas that were collected, the ideal gas formula PV = nrt is used
Procedure to calculate the number of moles of gasAssuming the normal conditions of temperature and pressure, we have the following data
n = ?
V = 50 cm3 = 0.05 L
R = 0.082 L atm / mol °K
t = 25°C = 298°K
P = 1 atm
n = PV / Rt
n = 1 x 0.05 / 0.082 x 298
n = 0.05 / 24,436
n = 0.002 mole
Learn more about moles of gas collected during reactions at https://brainly.com/question/1217205
#SPJ4
What are the effects of chemical exposure?
Answers
The effects of chemical exposure includes difficulty in breathing and blistering of skin.
Well we are working in a laboratory, there are a few certain ways in which a harmful chemical can harm us.
For an example, if any chemical is easily evaporated at the room temperature then there is a very high possibility that we may inhale the chemical along with air into our lung which make create difficulty in breathing.
Also some chemicals like concentrated hydrochloric acid or concentrated Sodium Hydroxide are the chemicals which can produce blistering of skin when they come in contact with the human skin.
The above stated effects are the consequences of chemical exposure.
To know more about chemical exposure, visit,
https://brainly.com/question/13722425
#SPJ4
Which model could represent a neutral atom of boron?
A. 4
B. 3
C. 1
D. 2
Answers
Answer: The option B. 3 is the answer.
Explanation:Boron has an Atomic mass of 11 and 5 protons and 6 Neutrons.
The Electrons are also in the correct positions to be a boron atom.
elect True or False for each statement: The solubility of gases in water decreases with increasing temperature Most solids are more soluble at higher temperature. Pressure has little effect on the solubility of liquids and solids because they are almost incompressible.
Answers
True, The solubility of gasses in water decreases with an increase in temperature.
What is solubility?
A substance's solubility is the greatest amount that will dissolve in a given amount of solvent at a given temperature. A specific solute-solvent combination's solubility is a defining trait, and the solubility of various compounds can vary significantly.
When temperature rises, molecules' vibrational kinetic energy increases, breaking intermolecular bonds and allowing gases to escape from the solution, the solubility of gases in water is inversely proportional to temperature.
Hence, the solubility of gasses in water decreases with increase in temperature.
To learn more about solubility from the given link:
https://brainly.com/question/9098308
#SPJ4
a 3.5kg object is accelerating at 2.2m/ while being pushed by a force of 10 n to the right across the floor
Answers
The next external force exerted on the papaya is 7.7 N.
What is force?Force is the power that changes the motion of the object. The external force that accelerated the papaya across the table must be resolved in this problem. We can solve the external force by applying the idea of Newton's first law of motion.
F = ma
where
F is the force, the unit is in Newtons (N)
m is the mass, the unit is in kg
a is the acceleration, the unit is in m/s²
Given that, m = 3.5 kg
a = 2.2 m/s²
F = ?
F = ma
Substitute the given data
F = (3.5 kg) (2.2 m/s²)
F = 7.7 N
Therefore, the external force of the papaya is 7.7 N.
To learn more about force, refer to the link:
https://brainly.com/question/26262858
#SPJ1
The question is incomplete. Your most probably complete question is given below:
What is the next external force exerted of the papaya?
What are bonds example?
Answers
Some Examples of primary bonds such as ionic bond,covalent bond and metallic bond;
-Example of an ionic bond is the formation of sodium fluoride, NaF, from a sodium atom and a fluorine atom
-Covalent bonds usually occur between nonmetals. For example, in water (H2O) each hydrogen (H) and oxygen (O) share a pair of electrons to make a molecule of two hydrogen atoms single bonded to a single oxygen atom.
-Metallic bonding, is especially common in minerals involving transition metals. Gold, silver, and copper are examples of minerals with metallic bonds.
Chemical Bond and its type :-
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds. The strength of chemical bonds varies considerably; there are "strong bonds" or "primary bonds" such as covalent, ionic and metallic bonds, and "weak bonds" or "secondary bonds" such as dipole–dipole interactions, the London dispersion force and hydrogen bonding. Strong chemical bonding arises from the sharing or transfer of electrons between the participating atoms.
There are three primary types of bonding: ionic, covalent, and metallic.
An ionic bond is formed when valence electrons are transferred from one atom to the other to complete the outer electron shell.
A covalent bond is formed when the valence electrons from one atom are shared between two or more particular atoms.
A metallic bond is formed when the valence electrons are not associated with a particular atom or ion, but exist as a "cloud" of electrons around the ion centres.
To know more about Chemical bond visit here ; https://brainly.com/question/12907148?referrer=searchResults
#SPJ4
which molecular orbital has zero nodes, contributes to bonding, and has nonzero electron density directly along the internuclear axis?
Answers
An entirely bonded molecular orbital has non-zero electron density, no nodes, and is perpendicular to the internuclear axis.
Molecular orbital-Molecular orbitals are linear combinations of atomic orbitals that represent the electron distribution across two or more atoms. A molecular orbital is the orbital or wavefunction of an electron in a molecule.
A mathematical function termed a molecular orbital describes the position and wave-like behavior of an electron inside a molecule.. You can compute chemical and physical attributes using this function. Electrons in the vicinity of a molecule can be associated with multiple atoms and are frequently expressed as a combination of atomic orbitals.
LEARN MORE ABOUT Molecular orbital HERE
https://brainly.com/question/17371976
#SPJ4
the gas in a 250.0 ml piston experiences a change in pressure from 1.00 atm to 3.45 atm. what is the new volume (in ml) assuming the moles of gas and temperature are held constant?
Answers
The new volume (in ml) assuming the moles of gas and temperature are held constant is 72.4 ml.
What is pressure?
A barometer or manometer can be used to measure pressure, which is the force applied per unit area. For an accurate physical description of a sample of a gas, four variables must be known: temperature, volume, quantity, and pressure. The SI unit for pressure is the pascal (Pa), which equals one newton per square meter (N/m²) of surface area. Pressure is defined as force per unit area of surface. An object's pressure is inversely proportional to the area on which the force is applied, and it is proportional to the force it exerts.
We have,
At constant temperature = P₁V₁ = P₂V₂
Where,
Initial pressure, P₁ = 1.00 atm
Final pressure, P₂ = 3.45 atm
Initial volume, V₁ = 250 ml
Final volume, V₂ = ?
Putting values in the formula
=(1.00 atm) × (250ml) = (3.45 atm) × V₂
V₂ = 72.4 ml
To know more about pressure, check out https://brainly.com/question/28012687
#SPJ4
the mass of a nitrogen molecule is 14 times greater than that of a hydrogen molecule. what is the temperature of a sample of hydrogen whose average molecular energy is equal to that in a sample of nitrogen at 300 k?
Answers
The kinetic strength of one mol of nitrogen molecules at three hundred K is 3741.3 Joules.The KE is 41 kj.
We recognize that,Kinetic strength ∝ TIn the question, temperature is equal in each conditions. So, the kinetic strength withinside the 2d situation is E.Room imply rectangular velocity is given by
vrms=√3RTM
Here,M= Molar mass of fueloline moleculeT= temperature of the fueloline moleculeWe have given VN2=VH2
∴√3RTN2MN2=√3RTH2MH2
⇒TH22=57328⇒TH2≈41 K
Read more about nitrogen:
https://brainly.com/question/1380063
#SPJ4
explain why real molecules like water and ammonia have bond angles of 104.5o and 107o instead of 109.5o.
Answers
Answer:
This is because of the lone pairs present in the molecules. Bond and lone pairs of electrons have maximum mutual repulsion but lone pairs of electrons have a stronger mutual repulsion than bond pairs thus creating a bent or v shape in water and trigonal pyramidal shape in ammonia.
There are 4 pairs of electrons in water. if all were bond pairs then the bond angle would be 109.5 but because of the the two lone pairs you take away 2.5 per lone pair from 109.5. Same goes to ammonia if they were all bond pairs then it would be 109.5 but because of the one lone pair it becomes 107.
Explanation:
five grams of nitrogen gas at an initial pressure of 3.0 atm and at 20∘c undergo an isobaric expansion until the volume has tripled.
a. What is the gas volume after the expansion?
b. What is the gas temperature after the expansion (in degrees Celsius)?
The gas pressure is then decreased at constant volume until the original temperature is reached.
c.What is the gas pressure after the decrease?
Finally, the gas is isothermally compressed until it returns to its initial volume.
d.What is the final gas pressure?
e. Show the full three-step process on a pV diagram. Use appropriate scales on both axes.
Answers
(a) The final volume after expansion is V2=4.29 L.
(b) The final temperature after the expansion is, T2=879K.
(c) The gas pressure after the decrease is P2=1atm.
(d) The final gas pressure is P3 = 3atm
Let V1 be the initial volume of the gas. Considering nitrogen as an ideal gas, use the ideal gas equation to find the initial volume as,
P1V1=nRT1...............(1)
Consider the value of universal gas constant as,
R=0.0821L⋅atm⋅mol⁻¹⋅K⁻¹.
Convert temperature to Kelvin,
T1=(20+273)K=293K
Consider the molar weight of nitrogen gas as, mm=28g/mole.
The number of moles in 5g of nitrogen gas is,
n=m/mm
Substitute the known values,
n=5g/(28g/mole)
=0.179mol
Substitute all the known and calculated values in equation (1),
V1=0.179mol×0.0821L⋅atm⋅mol−1⋅K−1×293K3atm/3 atm
=1.43L
B.
The final temperature after the expansion is, T2=879K.
The temperature after the expansion is evaluated using the formula,
V1/T1=V2/T2
T2=V2T1/V1
Substitute the known values,
T2=4.29L×293K/1.43L
=879K
C .
The gas pressure after the decrease is P2=1atm.
Using relation,
PV/T=constant
Since volume is kept constant,
P1/T2=P2/T3
P2=P1T3/T2
Substitute the known values,
P2=3atm×293K/879K
=1atm
D.
The final gas pressure is P3 = 3atm
Given data:
Gas is isothermally compressed to regain the initial volume, that is,
V3=1.43L .
Using relation,
PV/T=constant
Since temperature is kept constant,
P2/V2=P3/V3
P3=P2V2/V3
Substitute the known values,
P2=1atm×4.29L/1.43L
=3atm
The final gas pressure is P3=3atm
Pressure (symbol: p or P) is the force normal to the surface of an object per unit area over which that force is distributed.
Various units are used to express pressure. Some of these are units of force divided by units of area. For example, the SI unit of pressure, Pascal (Pa), is 1 Newton per square meter (N/m2). Similarly, pound-force per square inch (psi) is the traditional unit of pressure in imperial and US systems. Pressure can also be expressed as standard atmospheric pressure. Atmospheric pressure (atm) is equal to this pressure and torr is defined as 1/760 of this.
Therefore, manometric units such as centimeters of water, millimeters of mercury, and inches of mercury are used to express pressure as the height of a particular liquid column within a manometer.
Learn more about pressure here : brainly.com/question/25736513
#SPJ4
Using standard heats of formation, calculate the standard enthalpy change for the following reactions.
a. Fe3O4(s)+4H2(g)→3Fe(s)+4H2O(g)
b. C(s,graphite)+O2(g)→CO2(g)
c. NH2Cl(aq)→NH3(g)+HCl(aq)
Answers
The reaction between CH4(g) + 2O2(g) and CO2(g) + 2H2O(l) results in an enthalpy change of -891 kJ/mol.
When given the temperatures of formation, how can we determine the standard enthalpy change?The standard enthalpy change of formation, in this equation, is defined as the product of the standard enthalpies of formation of the reactants and the sum of the standard enthalpies of formation of the products. Along with the data for the normal formation enthalpy: H fo[A]=433 KJ/mol. H fo[B] = -256 KJ/mol. The reaction enthalpy can be calculated by subtracting the sum of the enthalpies of all the reactants from the sum of the enthalpies of the products. By subtracting the sum of the enthalpies of the reactants from the total enthalpies of the product, we may get the thermodynamic constant, or tH.To learn more about Enthalpy change refer to:
https://brainly.com/question/16387742
#SPJ4
What does half-life of 4 hours mean?
Answers
The duration needed for a drug's active ingredient to halve in your body is known as the half-life.
The duration needed for a drug's active ingredient to halve in your body is known as the half-life. According to how the body breaks down and eliminates the substance, this will happen. It can range from a few of hours to a couple of days, or even a couple of weeks. It may be possible for you to switch to a similar drug with a longer half-life if you are on a short-half-life medication and experiencing withdrawal symptoms. This medication with a longer half-life might be simpler to stop taking.
The duration of a drug's half-life can also serve as a reference for how long it will take for your body to adjust to its first dose and reach a steady level. It will typically take around.
Learn more about half-life here:
https://brainly.com/question/24710827
#SPJ4
How many total electrons must be transferred to form one formula unit of the compound al2o3?.
Answers
Six electrons are transferred in the formation of Al₂O₃.
Aluminum metal and Oxygen react to form Al₂O₃ as,
2 Al + 3/2 O₂ → Al₂O₃
Oxidation number of Al on left hand side is zero, while than on right hand side in Al₂O₃ is +3.
It means it has lost 3 electrons per one atom and six electrons per two atoms. Also, the oxidation number of O at left hand side in O₂ is zero, while that in Al₂O₃ it is -2 per atom and -6 per 3 atoms.
So, two Al atoms have lost 6 electrons and 3 O atoms have gained six electrons.
To look more about formula unit click here
brainly.com/question/21494857
#SPJ4
Answer:
6
Explanation:
Al has a left-side oxidation number of zero and a right-side oxidation number of three. means that it has lost three electrons for every single atom and six for every two atoms. Additionally, the left-hand side of O2 has an oxidation number of zero, whereas Al2O3 has an oxidation number of -2 per atom and -6 per three atoms. Thus, three O atoms have received six electrons whereas two Al atoms have lost 6 electrons.