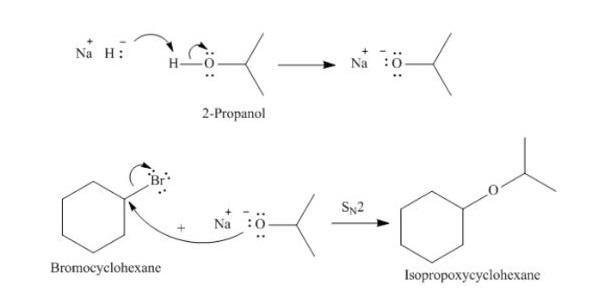
draw an alcohol and an alkyl bromide that could be combined to give the ether shown. an alkyl bromide and alcohol combine to form an ether. the ether is an oxygen bonded to a 6 carbon ring and isopropyl group. draw the alkyl bromide. draw the alcohol.
Answers
The product isopropoxycyclonehexane (ether) can be obtained from the following reaction of bromocyclohexane with 2-propanol in the presence of strong base
bromocyclohexane + 2-propanol ----(NaH/SN2) ---> isopropoxycyclonehexane
This occurs due to the strong base and the SN2 reaction.
Mechanism:
The strong base, sodium hydride abstracts a proton from 2-propanol to form an anion. This anion reacts with bromocyclohexane by SN2 mechanism to form isopropoxycyclonehexane.
Substitution reactions are those reactions in which one functional group is replaced by another functional group.
Two types of substitution reactions can occur in haloalkanes: SN1 and SN2.
Thus, the SN2 substitution reaction, in which the halogen is released from the haloalkane, and the nucleophile attacks the carbon's positive charge. SN2 in which the nucleophile attacks the halogen's carbon, causing the halogen to be replaced in which nucleophile attack.
To know more about SN2, refer: https://brainly.com/question/29589832
#SPJ4
Related Questions
Why do we produce formaldehyde?
Answers
We produce formaldehyde for medicinal purposes and synthesizing polyester.
Formaldehyde is a compound that is required in about 1.5 ounces in quantity as a normal part of our metabolism.
Also formaldehyde is used as a catalyst in the formation for the synthesis of many polyesters.
Formaldehyde also has many uses such as fungicide, germicide, disinfectant and as a preservative in mortuaries as well as important chemical in medical Laboratories.
In everyday items press fabric paints coating and paper base products also includes formaldehyde.
To know more about formaldehyde, visit,
https://brainly.com/question/29550668
#SPJ4
which of the compounds would most readily react with a strong base, such as nanh2, to form a carbanion?
Answers
NaNH2 is a strong base & excellent nucleophile. Benzene diazonium chloride when reacts with hypo phosphorus acid produces to form a carbanion.
What is base?
There are three common definitions of the term base in chemistry known as Arrhenius base, Bronsted base and Lewis base. All definitions are first given by G.-F. Ruel in the mid-eighteenth century.
Therefore, NaNH2 is a strong base & excellent nucleophile. Benzene diazonium chloride when reacts with hypo phosphorus acid produces to form a carbanion.
To learn more about base refer the given link:-
https://brainly.com/question/28907871
#SPJ4
what is the theoretical yield for this reaction in grams if you start with 0.240 ml of 1-methylcyclohexene and use the reagents in the amounts described in the procedure? report your answer to three decimal places but do not include the unit.
Answers
0.391 is the theoretical yield for this reaction in grams if we start with 0.240 ml of 1-methylcyclohexene and use the reagents.
Theoretical yield is the amount of a product that results from the complete conversion of the limiting reactant in a chemical reaction.
The maximum mass of a product that can be produced in a chemical process is known as the theoretical yield. The relative formula mass of the product, the mass and relative formula mass of the limiting reactant, and the balanced chemical equation can all be used to compute it.
A clear, colorless liquid with a smell resembling petroleum is what methylcyclohexane looks like. 25 °F flash point Water-insoluble and less dense than water.
To know more about mass, visit:
https://brainly.com/question/28021242
#SPJ4
A sample of gas weighs 3.33 g and occupies a volume of 1.365 l at 95 °c and 790 torr. identify the gas sample.
Answers
The gas sample is having molar mass of 64 g/mol
Identify the gas sample ?The question is incomplete, here is the complete question:
A sample of gas weighs 10.1 g and occupies a volume of 5.65 L at 60 °C and 580 torr. Identify the gas sample.
(a) (molar mass = 119.4 g/mol)
(b) (molar mass = 44.02 g/mol)
(c) (molar mass = 64.07 g/mol)
(d) (molar mass = 17.03 g/mol)
(e) (molar mass = 70.90 g/mol)
To calculate the molar mass of the gas, we use the equation given by ideal gas equation:
PV = nRT
P = Pressure of the gas = 580 torr
V = Volume of the gas = 5.65 L
w = Weight of the gas = 10.1 g
M = Molar mass of gas = ?
R = Gas constant =62.36 L
T = Temperature of the gas = 60 degree C = ( 60+ 273) K = 333K
Putting values in above equation, we get:
580 torr * 5.65 l = 10.1/ M* 62.364 L
M = 64g /mol
Hence, the gas sample is having molar mass of 64 g/mol
To learn more about gas sample refer
https://brainly.com/question/25736513
#SPJ4
what is the molar mass of potassium osmium manganese
Answers
The molar mass of the Potassium, Osmium, and Manganese are 39.10 g/mol, 190.23 g/mol, and 54.94 g/mol respectively.
What is the molar mass?The molar mass can be described as the mass of one mole of a substance. In one mole, there are 6.023 × 10²³ molecules or atoms present and this number is known as Avogadro’s number.
The molar mass of a chemical compound is determined by the sum of the atomic mass of each substance in the molecule.
The molar mass can be described as an intensive property of the substance as it does not depend on the size of the sample. In the S.I. system, the unit of molar mass can be represented as kg/mol. Molar masses are almost always represented in g/mol.
Learn more about molar mass, here:
brainly.com/question/12127540
#SPJ1
As a system reaches thermal equilibrium, _____ has been transferred from areas of high to low _____.
Answers
The Thermal energy is transferred from thermal equilibrium and that has been transferred from areas of high to low temperature.
As the temperature of an object increases, the average kinetic energy of its particles increases. As the average kinetic energy of its particles increases, the thermal energy of the object increases. Therefore, the thermal energy of an object increases as its temperature increases. 2.
Heat is the flow of energy from hot to cold. When these temperatures equalize, the heat flow stops and the system (or set of systems) shuts down. should be in thermal equilibrium. Thermal equilibrium also means that no matter enters or leaves the system.
Read more about heat;
http//brainly.com/question/934320
#SPJ4
a solution is prepared by bubbling nh3 in water until 25 g of nh3 dissolves. the final solution weighs 250 g and the density of the solution is 0.974 g/ml. what is the mole fraction of nh3 in the solution?
Answers
The mole fraction of the NH₃ in the solution is 0.0955.
given that :
mass of the NH₃ = 25 g
mass of the H₂O = 250 g
moles of the NH₃ = 25 g/ 17.03 g /mol
= 1.468 mol
moles of the H₂O = 250 g/ 18 g /mol
= 13.88 mol
total moles = 15.36 mol
the mole fraction of NH₃ = moles of NH₃ / total moles
= 1.468 / 15.36
= 0.0955
Thus, A solution is prepared by bubbling NH₃ in water until 25 g of nh3 dissolves. the final solution weighs 250 g and the density of the solution is 0.974 g/ml. the mole fraction of NH₃ in the solution is 0.0955.
To learn more about mole fraction here
https://brainly.com/question/15883465
#SPJ4
a chemist, running tests on an unknown sample from an illegal waste dump, isolates 18 grams of what he suspects is a radioactive element. in order to help identify the element, he would like to know the half-life. he determines that after 50 days only 5 grams of the original element remains. what is the half-life of this mystery element? round your answer to two decimal places, if necessary.
Answers
The half-life. he determines that after 50 days only 5 grams of the original element remains The half-life of this mystery element 26.99 days.
The expression for the half life is given as :
5 = 18(1/2)^50/t
log( 5/18 ) = 50 / t log( 1/2)
-0.5575 = 50 / t ( - 0.3010)
t = 15.05 / 0.5575
t = 26.99 days
Thus, A chemist, running tests on an unknown sample from an illegal waste dump, isolates 18 grams of what he suspects is a radioactive element. in order to help identify the element, he would like to know the half-life. he determines that after 50 days only 5 grams of the original element remains. the half-life of this mystery element is 26.99 days.
To learn more about half life here
https://brainly.com/question/6770013
#SPJ4
place the components of the electron‑transport chain to outline the flow of electrons from nadh to o2.
Answers
The components of the electron‑transport chain to outline the flow of electrons from NADH to O₂ is :
NADH --> NADH dehydrogenase ---> ubiquinone ---> cytochrome b-c1 complex ----> cytochrome c ---> cytochrome oxidase complex ---> oxygen ( O₂ ).
There are three respiratory enzymes complex in the respiratory chain in the inner mitochondrial membrane. The electron transport chain passes on the way from NADH to O₂ n order as given below:
NADH --> NADH dehydrogenase ---> ubiquinone ---> cytochrome b-c1 complex ----> cytochrome c ---> cytochrome oxidase complex ---> oxygen ( O₂ ). in the electron transport chain the oxygen is the final electron acceptor. the NADH is donor of the electrons.
To learn more about electron transport here
https://brainly.com/question/29432698
#SPJ4
What is the best salt to use on roads?
Answers
Answer:
Sodium Chloride
Explanation:
The most common substance used for deicing roads and highways is Sodium Chloride (NaCl) or table salt known as rock salt when spread on the road because of its much larger granules.
60 POINTS ILL GIVE BRAINLIEST PLEASE HELP
Answers
Brainliest is appreciated
the volume of 350. ml of gas at 25°c is decreased to 135 ml at constant pressure. what is the final temperature of the gas?
Answers
The decrease in the volume of gas at constant pressure results in the final temperature of the gas is 115.05 K.
The Charles law states that with the gas constant pressure there has been a proportional relationship between the volume and temperature.
Gas is a state of matter that has no fixed shape and no fixed volume. Gases have a lower density than other states of matter, such as solids and liquids. There is a great deal of empty space between particles, which have a lot of kinetic energy and aren't particularly attracted to one another.
Kinetic electricity is the energy an object has due to its movement. If we need to boost up an item, then we ought to follow a pressure. applying a force calls for us to do paintings. After work has been achieved, energy has been transferred to the item, and the object might be shifting with a brand new consistent pace.
Learn more about gas here :-
brainly.com/question/24719118
#SPJ4
The following ions contain the same number of electrons. Rank them in order of decreasing ionic radii!
Sc3+, P3-, Cl-, Ca2+, K+, S2-
Answers
The ionic radii for the given ions are respectively described:
[tex]Sc^{3+}[/tex] = 74.5 pm
[tex]P^{3-}[/tex] = 212 pm
[tex]Cl^{-}[/tex] = 181 pm
[tex]Ca^{2+}[/tex] = 100 pm
[tex]K^{+}[/tex] = 138 pm
[tex]S^{2-}[/tex] = 184 pm
Therefore, to put them in decreasing order according to the ionic radii, then the order would be:
[tex]P^{3-}, S^{2-}, Cl^{-}, K^{+}, Ca^{2+}, Sc^{3+}[/tex]
The radius of a single ion in an ionic crystal is denoted by the symbol "[tex]r_{ion}[/tex]." The distance between ions in a crystal lattice is equal to the sum of the radii of the cation and anion, despite the fact that neither atoms nor ions have clear boundaries. The radius of an ion is commonly expressed in picometers (pm) or angstroms (Å), where 1 Equals 100 pm.
Coordination number, spin state, and other characteristics affect ionic radius. Ionic radius readings are transferrable enough to identify periodic trends. Ionic radii rise with group descent, like other atomic radii. High-spin ions are larger than low-spin ones, and coordination number enhances ionic size. Positive charge decreases ionic radius, while negative charge increases it.
To know more about ionic radius:
https://brainly.com/question/8137711
#SPJ4
9.0 mol Na2S reacts with 8.0 mol
CuSO4 according to the equation below:
Na2S + CuSO4 → Na2SO4 + CuS
Considering only the 9.0 mol Na2S, how
many moles of CuS can form?
?] mol CuS
Answers
Here, CuSO₄ is the limiting reagent. Considering only the 9.0 mol Na₂S, 1 mole of CuS can form.
What is limiting reagent?The limiting reagent is the one that is completely consumed during a reaction. Reagents that are entirely consumed by a chemical reaction are known as limiting reagents. They are additionally known as limiting reactants or limiting agents.
The limiting reagent is the reactant which completely reacts before the other reactant(s) is used up. When 9.0 moles Na₂S and 8.0 moles CuSO₄ react, it appears that CuSO₄ is the limiting reagent.
Now, no. of moles of CuS = (mass of substance) / (mass of one mole)
= (95.611 g/moles) / (95.611 g/moles)
= 1 moles.
To know more about limiting reagent refer to:
https://brainly.com/question/26905271
#SPJ1
Choose the combination of factors that would lead to the greatest oxygen unloading from hemoglobin.
a. Low pH, high temperature, high Pcoz, high 2,3-BPG b. Low pH, high temperature, low Pcos, high 2,3-BPG High pH, c. low temperature, low Pcoz, low 2,3-BPG d. High pH, high temperature, high Pcoz, low 2,3-BPG
Answers
The combination factors that would lead to greatest oxygen unloading from hemoglobin is Low pH, high temperature, high PCO2, high 2,3-BPG.
Group 16 of the periodic table, also known as the oxygen group, contains the non-metallic chemical element oxygen (O). All living things require oxygen, a colorless, flavorless, and odorless gas. Plants utilize the carbon dioxide that animals have absorbed and converted into by using it as a source of carbon and releasing the oxygen back into the environment. In addition to reactions that remove elements from their combinations with one another, oxygen can react with almost every other element to form compounds. These reactions are referred to as combustions, and they frequently result in the production of heat and light. Water is the most crucial component.
To know more about oxygen visit : brainly.com/question/11587330
#SPJ4
why did we choose these particular salts to test how the soap reacts with hard water?
Answers
Epsom Salt is used to test how the soap reacts with hard water because it forms precipitates with hard water
Epsom salt also known as magnesium sulfate is used to check the reaction of soap with hard water because it separates into ions giving us Mg2+ and SO4 2- ions.
Now, when we add soap to this water, soap combines with the magnesium ions and form solid particles that accumulate in clusters that become insoluble. These clusters are called precipitates which can be observed physically.
Hence, it proves the hardness of water and explains why particular salts to test how the soap reacts with hard water.
You can learn more about how soap reacts with hard water from
https://brainly.com/question/29823234
#SPJ4
Which of the following is a sign that a chemical change has occurred?
Group of answer choices
glass changes shape when it falls and breaks
water produces a gas when it is placed on a hot stovetop
a firework explodes releasing heat, sound, and light energy
a substance becomes cold when it is placed in the freezer
Answers
The national fire protection association (nfipa) 704 diamond label system helps firefighters and employees easily recognize the hazardous substances stored on the site.
Answers
The (NFIPA) 704 diamond label system helps firefighters and employees easily recognize the hazardous substances stored on the site is correct statement.
The categories of hazardous materials stored on the site are easily recognised by firefighters and staff thanks to National Fire Protection Association (NFIPA) 704 standards. The National Fire Protection Association has published it to supplement the labelling system for emergency responders and to help those who work in regular environments. Materials Safety Data Sheet refers to the document created by the manufacturer that lists the risks associated with a product. Hazardous materials are defined as substances or materials that, when transported, stored, or used in commerce, could pose an unreasonably high risk to one's health, safety, or property.
To know more about National Fire Protection Association visit : https://brainly.com/question/11251534
#SPJ4
pressure has little effect on the solubility of liquids and solids because they are almost incompressible. t or f
Answers
False. Pressure has a direct impact on the solubility of liquids and solids. Increasing pressure raises the solubility of a given substance, while decreasing pressure reduces it. This is because higher pressure forces molecules closer together, making it easier for them to dissolve in a solvent.
The Impact of Pressure on the Solubility of Liquids and SolidsThe effect of pressure on the solubility of liquids and solids is an important factor to consider in many industrial processes. When the pressure is increased, the solubility of a given substance increases as well, due to the increased force between molecules which makes it easier for them to dissolve in a solvent.
Learn more Solute and solvent: https://brainly.com/question/25326161
#SPJ4
Arrange the organic compounds from most soluble in water to least soluble in water.
a. CH4 b. CH3OH c. CH3OCH
Answers
The substance that is most soluble in water among the other substances is ethylene glycol (HOCH2CH2OH). The two hydroxy groups in ethylene glycol combine with water to produce hydrogen bonds.
Which organic molecule dissolves the least easily in water?
Octane is the chemical compound that dissolves the least readily in water. Because only two hydrocarbons, carbon and hydrogen, make up octane, it is a nonpolar molecule due to the comparable electronegativities (ENs) of the atoms. The least soluble fuel in water will be octane because water is a polar solvent.
Is CH3OCH3 water soluble?
Dimethyl ether (CH3OCH3) (C H 3 O C H 3) and water molecules also form hydrogen bonds. As a result, it is also soluble in water, but due to the bulky methyl group in dimethyl ether, there is less hydrogen bonding.
To know more about soluble in water visit;
https://brainly.com/question/5173732
#SPJ4
Using the following equation for the combustion of octane, calculate the heat of reaction for 50.00 g of octane. The molar mass of octane is 114.33 g/mole.
2 C8H18 + 25 O2 → 16 CO2 + 18 H2O ΔH∘rxn = -11018 kJ
Using the following equation for the combustion of octane, calculate the heat of reaction for 50.00 of octane. The molar mass of octane is 114.33 .
Answers
When the heat of reaction is applied to 50 octane, the heat of reaction is -2407.43 KJ. Octane has a molar mass of 114.33.
Explain about the octane?It's important not to confuse octane with the octane number assigned to gasoline because octane is a hydrocarbon with an eight-atom chain. The antiknock characteristics of fuels are compared using the octane rating system, which uses a scale with 0 being pure n-heptane and 100 being pure 2,2,4-trimethylpentane (isooctane, an isomer of octane).
When a fuel is burnt in a mixture with air in the cylinder of an internal combustion engine, its capacity to withstand knocking is measured by its octane number, often known as its antiknock rating.
The liquid n-octane has the smell of gasoline and is colorless. Both less dense and insoluble in water as a result, floats on water. In order for modern engines to operate correctly, octane, a fuel additive, is required.
Octane weight is 50 g.
Octane's molecular weight is 114.33 g/mole.
We must first determine the octane molecular weight.
= 50/114.33g/mole.
=0.4373
Heat energy released by 2 moles of octane is -11018 KJ.
Octane releases 0.437 moles of heat energy, so
-11018/2 x s 0.437
=-2407.433
To learn more about octane refer to:
https://brainly.com/question/28469125
#SPJ4
Sulfur (s) and fluorine (f2) react to form sulfur hexafluoride (sf6). If 50. 0 g s reacts with 105. 0 g f2, what mass of the excess reactant remains?.
Answers
The mass of extra reagent left is 20.58 g of the Sulfur (s) and fluorine (f2) react to form sulfur hexafluoride (sf6).
To calculate the quantity of moles, we use the equation:
Number of moles Given mass Molar massFor sulfur:Given mass of sulfur = 50 gMolar mass of sulfur = 32q / molPutting values in equation 1, we get:Moles of sulfur 50g 32g/molFor fluorine fueloline:Given mass of fluorine fueloline = 105gMolar mass of fluorine fueloline = 37.99 g/molPutting values in equation 1, we get:Moles of fluorine fueloline 2.76mol= (105g)/(37.99g / mol) = = 1.56molThe chemical equation for the response of sulfur and fluorine fueloline follows:S(g) + 3F(g) -> SF(g)By Stoichiometry of the response:three moles of fluorine fueloline reacts with 1 mole of sulfurSo, 2.seventy six moles of fluorine fueloline will react with = 1 × 2.76 = 0.92mol of sulfurAs, given quantity of sulfur is greater than the specified quantity. So, it's far taken into consideration as an extra reagent.Thus, fluorine fueloline is taken into consideration as a restricting reagent as it limits the formation of product.Moles of extra reagent (sulfur) left = 1.56 0.92 0.64 moles.Now, calculating the mass of sulfur from equation 1, we get:Molar mass of sulfur = 32g / molMoles of sulfur = 0.sixty four molesPutting values in equation 1, we get:0.64mol = (Massofsulfur)/(32g / mol)Massofsulfur = 20.48gHence, the mass of extra reagent left is 20.48 gRead more about Flourine:
https://brainly.com/question/15045637
#SPJ4
What reacts quickly with bromine solution?
Answers
They react rapidly with bromine, for example, to add a Br2 molecule across the C=C double bond. This reaction provides a way to test for alkenes or alkynes.
A flask has a mass of 78. 23 g when empty and 593. 63 g when filled with water. When the same flask is filled with concentrated sulfuric acid, h2so4, its mass is 1026. 57 g. What is the density of concentrated sulfuric acid in g/cm3? (assume water has a density of 1. 00 g/cm3 at the temperature of the measurement. ).
Answers
The density of concentrated sulfuric acid that filled into a flask with 1026.57g mass is 1.84 g/cm^3.
It is the substance's mass per unit of volume. The symbol that used for density is ρ and it expressed in units of grams per cubic centimetre.
How to calculate the density of concentrated sulfuric acid, H2SO4?
Volume of Flask = 593.63 - 78.23 = 515.4g of water
1g of water = 1cm^3 so the volume is 515.4cm^3
Mass = 1026.57 - 78.23 = 948.34g
Density of sulfuric acid = Mass / Volume
= 948.34 / 515.4
= 1.84 g / cm^3
Therefore, the density of concentrated sulfuric acid is 1.84 g/cm^3
Learn more about density https://brainly.com/question/952755
#SPJ4
The compound SrBr₂ contains how many atoms?
02
04
01
0 3
Answers
One Strontium atom and two Bromine atoms
Hope this helps. Please give brainliest
what is a sign in the chemical reaction that the acid/base is stronger than the conjugate acid/conjugate base in a chemical reaction?
Answers
If the acid and base are the reactants, then the conjugate acid and conjugate base are the products
What is Conjugate Acid?
There is a conjugate base for every acid. Every base has an acid conjugate. When acids react, they "give" H+. When they separate in water, this is most clearly visible:
This illustration uses sulfuric acid (H
Due to the fact that it "donates" H + to the water, 2 S O 4) is an acid. It turns into the hydrogen sulfite ion, or H S O 4—the conjugate base of sulfuric acid.
A conjugate acid differs from the base it was generated from in that it has one additional H atom and one more + charge
The conjugate base is what is left over after the acid has finished doing its work and lost a proton.
The conjugate acid is what is produced after the initial base has finished its work. take in a proton.
It truly is that easy.
KOH + HCl results in K+, Cl-, and H2O.
Cl(-) is the conjugate base of HCl in the example above, while water is the conjugate acid of hydroxide.
Learn more about Conjugate Acid from given link
https://brainly.com/question/12584785
#SPJ4
what is required in order to be able to identify an element based on its flame test color
Answers
In the flame test, the metal is put under the flame. The flame turns in different colors based on which the element is identified.
What is the flame test?A flame test can be described as an analytical procedure used in chemistry to detect the presence of elements, primarily metal ions, based on the characteristic emission spectrum of each element. The color of flames depends on temperature and oxygen.
This test involves introducing a sample of the compound to a hot, non-luminous flame, then observing the color of the flame that results. Sample atoms evaporate when they are hot, they release light when being in flame.
Bulk samples can emit light too, but their light is not good for analysis. Bulk samples emit light with HCl to remove traces of previous analytes.
Learn more about the flame test, here:
https://brainly.com/question/6357832
#SPJ1
the atmospheric pressure at the surface of venus is 90.8 atm. the venusian atmosphere is 96.5% co2 and 3.5% n2 by volume, with small amounts of other gases also present. compute the mole fraction and partial pres- sure of n2 in the atmosphere of venus.
Answers
The proportion of nitrogen and oxygen in our atmosphere is roughly 78% and 21%, respectively, with smaller amounts of other gases making up the remainder. In a given sample of air, nitrogen accounts for 78% of the gas particles and hence exerts 78% of the pressure.
Venus' atmosphere differs significantly from Earth's. 96.5 percent of the atmosphere's gases are carbon dioxide, and 3% are nitrogen. The abundance of nitrogen on Venus adds to pressure levels well over 2700mmHg because Venus' atmospheric pressure is around 92 times greater than Earth's. Humans could not breathe there because there is no oxygen there. Not that anyone would want to visit Venus because its surface is typically warmer than 460oC.
The Law of Partial Pressures of Dalton:
Gas pressure is the outcome of gas particle collisions with the inside walls of their container. The gas pressure rises as additional gas is supplied to a rigid container. It doesn't matter what the two gases are called. English chemist John Dalton, who made thewho developed the atomic hypothesis also looked at gas mixes. He discovered that each gas in a combination exerts pressure on its own, independent of the other gases.
For instance, The pressure of just the nitrogen in the air is 0.78 atm, if the total atmospheric pressure is 1.00 atm. The oxygen content of the air is 0.21 atm.
To know more about partial pressure, please refer:
https://brainly.com/question/14119417
#SPJ4
draw the alkyne formed when 2,3‑dichloropentane is treated with an excess of strong base such as sodium amide.
Answers
The same catalysts used in alkene hydrogenation—platinum, palladium, nickel, and rhodium—are utilized in the catalytic hydrogenation of alkynes. Step-by-step, hydrogenation creates an alkene first, which is then further hydrogenated to create an alkane.
How are alkenes created?
Elimination processes, which remove two atoms from adjacent carbon atoms to create a double bond, are typically used to create alkenes. Dehydration of alcohols, dehydrohalogenation of alkyl halides, and dehalogenation of alkanes are all steps in the preparation process.
What is an alkyne's end product?
Similar to alkenes, the major reaction route for alkynes is "addition," which entails rupturing the C-C bond and creating two new single bonds to carbon. Alkenes are the end product of addition reactions to alkynes, and as we just saw, addition reactions also occur in alkene reactions.
To know more about Alkynes visit;
https://brainly.com/question/23508203
#SPJ4
Is water an element
Answers
Answer:yes
Explanation:the element of the world is water,fire,air,and earth
It contains hydrogen and oxygen atoms and thus is not an element