Answers
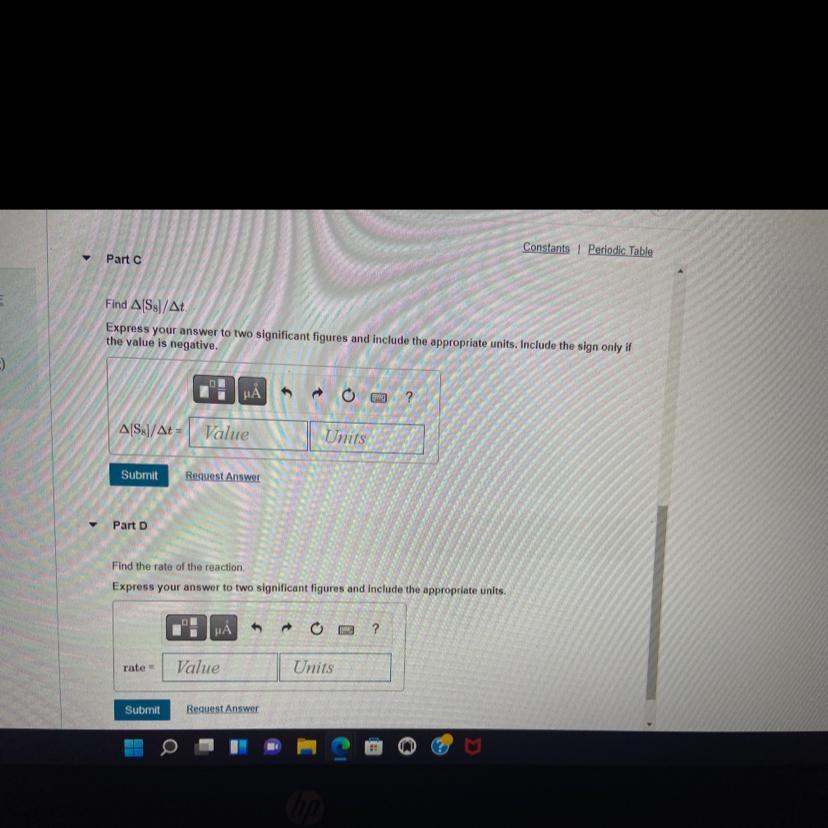
Part C the answer is Δ[S8]/Δt = 0.0026 M/s
Parte D the answer is v = 0.0026 M/s
Related Questions
2.8g of silicon react with 3.2g of oxygen to give a compound, which is shown below. The relative atomic mass of silicon is 28 and of oxygen is 16.What is the value of y in the formula below? SIOy
Answers
The value of Y in the formula when 2.8g of silicon react with 3.2g of oxygen, is 2
The numbers in the formula of a compound determine the ratio of moles of each element in the compound.
Given:
2.8g of silicon
3.2g of oxygen
[relative atomic mass of silicon is 28 and of oxygen is 16]
For silicon, n = 2.8 ÷ 28 = 0.1 (n= moles)
For oxygen, n = 3.2 ÷ 016 = 0.2
For the mole ratio, we divide each value of the moles by the smallest number of moles calculated which is 0.1 moles.
For Silicon = 0.1/0.1 = 1
For Oxygen = 0.2/0.1 = 2
Taking the mole ratio as their subscripts.
The ratio of Si:O is 1:2
SiO[tex]_{2}[/tex] is the formula of silicon dioxide.
The value of y in the formula SiO[tex]_{Y}[/tex] is 2 when 2.8g of silicon react with 3.2g of oxygen.
To know more about moles, click on this link
https://brainly.com/question/22540912
. In the reaction H₂ + I₂ --> 2HI, the iodine undergoes ______________________ (1 pt)
*
1 point
oxidation
reduction
no change in oxidation number
Answers
In the reaction H₂ + I₂ --> 2HI, the iodine undergoes reduction.
The balanced reaction as:
H₂ + I₂ --> 2HI
The oxidation state of hydrogen is increases from 0 to + 1
The oxidation state of iodine is decreases from 0 to -1
The increase in oxidation number is called oxidation. The decrease in oxidation number is called as reduction . the loss of electrons is called as oxidation and the gain of electron is called as reduction
oxidation = hydrogen , reducing agent = hydrogen
reduction = oxygen , oxidizing agent = oxygen
Thus, In the reaction H₂ + I₂ --> 2HI, the iodine undergoes reduction.
To learn more about reduction here
https://brainly.com/question/28813812
#SPJ1
2C8H18(I) + 25 O2 (g) = 16 CO2(g) + 18 H2O (g)If 4.00 moles of gasoline are burned, what volume of oxygen is needed if the pressure is 0.953 atm and the temperature is 35 C?
Answers
Answer
Volume of O2 = 1326 L
Explanation
Given:
2C8H18(I) + 25 O2 (g) = 16 CO2(g) + 18 H2O (g)
moles of gasoline = 4.00 mol
Pressure = 0.953 atm
Temperature = 35 C = 308 K
Required: volume of oxygen
Solution
Step 1: use stoichiometry to find the moles of oxygen
The molar ratio between gasoline and O2 is 2:25
Therefore the moles of o2 = 4.0 mol x (25/2) = 50.0 mol
Step 2: Calculate the volume using ideal gas law
PV = nRT
V = nRT/P
V = (50.0 x 0,08206 x 308)/0.953
V = 1326 L
How do the four properties of liquids relate to the polarity of a molecule?Include the following terms in your answer: bonds, polar, non-polar, adhesion, cohesion,surface tension, capillary rise. This summary should be several sentences long. Needs to be a short summary including those words. Please HELP!
Answers
Answer
In summary
A number of properties of liquids, such as cohesion and adhesion, are influenced by the intermolecular forces within the liquid itself. Cohesion are various intermolecular forces that hold solids and liquids together while adhesion is the ability of a liquids to stick to an unlike substance. The rise or fall of a liquid in a capillary tube is governed by the balance of cohesive and adhesive forces. Lastly, surface tension is a fundamental property of the surface of liquid, it is responsible for the curvature of the surfaces of liquids dues to the polarity that exists in the liquid molecules.
Explanation
Liquids flow because the intermolecular forces between molecules are weak enough to allow the molecules to move around relative to one another. The forces are attractive when a negative charge interacts with a nearby positive charge and repulsive when the neighboring charges are the same, either both positive or both negative. Molecules are held together by polar covalent bonds – which means that the electrons are not evenly distributed between the bonded atoms. This uneven distribution in the covalent bonds of the molecules results in a partial charge.
The liquid molecules don’t interact particularly strongly with each other because the intermolecular forces are weak. The primary intermolecular forces – are London dispersion forces, which for small molecules are the weakest types of intermolecular forces. These weak forces lead to low cohesion. The molecules don’t interact strongly with each other, so they can slide right past one another.
Adhesion is the tendency of a compound to interact with another compound. (Remember that, in contrast, cohesion is the tendency of a compound to interact with itself.) Adhesion helps explain how liquids interact with their containers and with other liquids. One example of an interaction with high adhesion is that between water and glass. Both water and glass are held together by polar bonds. Therefore, the two materials can also form favorable polar interactions with each other, leading to high adhesion.
Based on the model, can hydrogen be used to make oxygen? and how do you know?
Answers
Answer:
can hydrogen be used to make oxygen? and how do you know?
Explanation: yes because H1 is water
Answer:
see the answer
Explanation:
Yes hydrogen can be used to make oxygen
because H1 is water
Which of the following is the best description of an oxidation process?Question 3 options:A) Oxidation is the non-spontaneous loss of electronsB) Oxidation is the spontaneous loss of electronsC) Oxidation is the gain of electronsD) Oxidation is the loss of electrons
Answers
INFORMATION:
We have the next options:
A) Oxidation is the non-spontaneous loss of electrons
B) Oxidation is the spontaneous loss of electrons
C) Oxidation is the gain of electrons
D) Oxidation is the loss of electrons
And we must select the one that best describes an oxidation process
STEP BY STEP EXPLANATION:
Oxidation process consists in the loss of electrons during a reaction.
An older meaning of oxidation was when oxygen was added to a compound. This was because oxygen gas (O2) was the first known oxidizing agent. While the addition of oxygen to a compound typically meets the criteria of electron loss and an increase in the oxidation state, the definition of oxidation was expanded to include other types of chemical reactions.
Finally, knowing that, we can state that, in general, Oxidation is the loss of electrons.
ANSWER:
D) Oxidation is the loss of electrons
How many moles of neon gas will occupy a volume
of 875 mL at 3.25 atm and 25°C?
Answers
The molarity of the solution is 2.33 mol /L
molarity is a physical quantity that describes the substance's number of moles available in the unit liter volume of the solution. A mole per liter is a measurable unit to explain molarity.
Number of moles of the NaCl is
np = 3.50 mol
The volume of the solution is np = 1.50 L
The expression for the molarity solution is cm = np/vp
At the value and get the above expression
Cm = 3.50/ 1.50
= 2.33 mol /L
Thus the molarity of the solution is 2.33 mol /L
To know more about molarity related questions click
https://brainly.com/question/26873446
#SPJ1
assuming you start with 1.25 g of pure aluminum, calculate the following: 1) the amount of potassium hydroxide, k o h , in grams, needed to react with all of the 1.25 g of a l . the reaction is:
Answers
The amount of mass potassium hydroxide (KOH) required to react with every 1.25 g of aluminum is 2.58 g.
The amount of mass potassium hydroxide (KOH) required can be calculate as follow:
as we know;
Mass of pure aluminum = 1.25g
Molar mass of KOH = 56 g/mol
The chemical reaction of a given element is given by:
[tex]2Al + 2 KOH + 6H_2O = > 2K^{+} +2Al(OH)^{4-} +3 H^{+}[/tex]
From the above reaction, we can deduce that:
2 mol AL ----- 2 mol KOH
How many moles of aluminum are needed for 1.25g?
[tex]n Al =\frac{ g}{mw} \\n Al =\frac{ 1.25}{27}\\nAl = 0.046 moles[/tex]
The mass of KOH required for 0.046 moles of aluminum is calculated as:
Mass Required = 0.046 x 56
Required mass = 2.58 g
Therefore, the amount of mass potassium hydroxide (KOH) required to react with every 1.25 g of aluminum is 2.58 g.
Learn more about mass potassium hydroxide here:
Brainly.com/question/13763130
#SPJ4
ionic bond formation by correctly pairing these terms: cation, anion, electron gain, and electron loss.
Answers
Answer:
cation - electron loss hence positive charge
anion - electron gain hence negative charge
The given terms are correctly paired thus: anion is to electron gain while cation is to electron loss.
What is ionic bond formation?Ionic bond formation is defined as the formation of an ionic bond during a chemical reaction whereby an atom losses electrons while another gains electrons through transfer of these electrons.
For example in the formation of the compound NaCl. Sodium is the element that donates an electron from its outermost shell to chloride. Therefore, the sodium atom is the cation.
Also the chloride element would accept electron from the sodium, therefore, it is called the anion.
Learn more about ionic bond here:
https://brainly.com/question/28894350
#SPJ1
pls answer question will mark brainliset tyty
Answers
Answer:
Answer is C,
Explanation:
It diminished local resources, decreasing the population.
Create a table showing how each type of radiation
affects the atomic number and mass number of an
atom.
Answers
The emitting element's atomic number and atomic mass number are both reduced by 2 and 4 respectively by alpha radiation. The atomic number of an element is increased by 1 by beta radiation, but the atomic mass number is unaffected. The atomic number or the atomic mass number are unaffected by gamma radiation.
What is radiation ?An unstable atom will release energy on its own to move into a more stable state, which is radioactivity. The energy that is released by a radioactive atom is known as ionizing radiation. The term "radioactive isotope" refers to radioactive atoms of the same element with varying neutron counts.
Alpha radiation decreases the atomic number and atomic mass number of the emitting element by 2 and 4 correspondingly. Beta radiation raises an element's atomic number by 1, but it has no effect on its atomic mass number. Gamma radiation has no impact on the atomic number or atomic mass number.
The atomic or mass number are unaffected by the emission of gamma rays, however the nucleus will become more stable. The most significant change in atomic number and mass number is brought on by alpha radiation.
A table showing how each type of radiation affects the atomic number and mass number of an atom is attached in the image below.
Thus, The emitting element's atomic number and atomic mass number are both reduced by 2 and 4 respectively by alpha radiation.
To learn more about radiation, follow the link;
https://brainly.com/question/13934832
#SPJ1
What is the name for N2P3?
a) Triphosphorus Dinitride
b) Dinitrogen Triphosphide
c) Nitrogen Monophosphide
d) Nitrogen Phosphide
Answers
Answer:
Dinitrogen trioxide (triphosphide)
Explanation:
31.The following equation represents which of the following types of reaction?4 H3PO4 ---> P4 + 5 O2 + 6 H2OSelect one:a. Decomposition.b. Double replacement.c. Single replacement.d. Synthesis.
Answers
Answer
A. Decomposition.
Explanation
A decomposition reaction can be defined as a chemical reaction in which one reactant breaks down into two or more products.
Atomic Structure Online Practice
Complete this assessment to review what you've learned. It will not count toward your grade.
The element phosphorous (P) has an atomic number of 15 and an atomic mass of 31. How many neutrons are in an atom of
phosphorous (P)? (1 point)
O 15
O 16
O 31
O 46
Answers
The correct answer is 16 that is the element Phosphorous has 16 neutrons.
The neutron is known as a subatomic particle that is slightly heavier than a proton and has a neutral charge (one that is neither positive nor negative). Atomic nuclei consist of both protons as well as neutrons.
Protons and neutrons both are referred to as "nucleons" because of how similarly they function inside the nucleus and because they both have masses that are about equal to one atomic mass unit.
Nuclear physics explains their interactions and characteristics. Each proton and neutron is made up of three quarks, hence they are not fundamental particles.
The arrangement of electrons around an atom's heavy nucleus mostlydetermines its chemical characteristics. The atomic number, or we can say number of protons, which determines the charge of the nucleus, determines the electron configuration.
To know more about 'Phosphorus' related questions
visit- https://brainly.com/question/4622631
#SPJ9
What happens when the seafloor spreads?
(A) Old crust moves closer together.
(B) New crust forms near the continental slope.
(C) New crust moves away from the mid-ocean rift.
(D) Basalt cools to form a new crust near the mid-ocean rift.
hurry im on a TEST!!!!
Answers
When the seafloor spreads Basalt cools to form a new crust near the mid ocean rift.
Seafloor spreads means the separation of seafloor. Tectonic plate and the large plate of the lithosphere will split apart from the each other. This process occurs at the mid ocean ridges and . seafloor spreading leads to dangerous things like rising sea level and earthquake . the cause for the seafloor spreading is ocean floors ruggedness. hot magma cooled to form igneous rock. this Basalt (the rock ) cools to form a new crust near the mid ocean rift.
Thus, When the seafloor spreads Basalt cools to form a new crust near the mid ocean rift.
To learn more about seafloor spread here
https://brainly.com/question/13732004
#SPJ1
The particles in which type of substance can be separated by physical
means?
A. Compound
B. Element
C. Mixture
D. Molecule
Answers
Answer:
Mixture
Explanation:
Mixture are simply a physical combination of two or more substances. they can be separated based only by physical means, or by undergoing physical changes.
Iodine-131 (I-131) is used in the treatment of thyroid cancer. It undergoes beta decay and has a half-life of 8 days.
If we start with 115 grams of I-131, how much will we have after 40 hours?
Answers
Iodine-131 (I-131) is used in the treatment of thyroid cancer it undergoes beta decay and has a half-life of 8 days if we start with 115 grams of I-131 then we have after 40 hours is 0.058g
Iodine-131 is the radioactive isotope of iodine with the atomic mass 131 and it is used for the treatment of thyroid cancer
Here given data is
half-life = 8 days
15 grams of I-131
We have to calculate after 40 hours = ?
So, m = M/2ⁿ
Then m = 15 grams/2⁸
m = 15 grams/256
m = 0.058g
Therefore If we start with 115 grams of I-131 then 0.058g will we have after 40 hours
Know more about Iodine-131
https://brainly.com/question/14470440
#SPJ1
How can a reaction generally be made to go more quickly?Check all that apply.A.Cooling the reactionB.Adding a catalystC.Lowering the activation energyD.Shaking the reactants
Answers
Answer
A reaction generally can be made to go more quickly by:
B. Adding a catalyst
C. Lowering the activation energy
D. Shaking the reactants
Explanation
The presence of a catalyst increases the rate of a reaction by lowering its activation energy. Chemical reactions occur when molecules collide with each other and undergo a chemical transformation.
Stirring and shaking provide some kinetic energy to the system. It increases slightly its temperature and the reaction rate. increasing the kinetic energy of reactant particles also means more of the reactant particles will have the minimum amount of energy required to form products (ie, activation energy) which leads to more successful collisions and therefore increases the reaction rate.
A 17. 5 ml portion of a 0. 5010 m na2co3 solution is added to 46. 0 ml of 1. 1250 m nacl. What is the concentration of sodium ion in the final solution?.
Answers
The concentration of sodium ions in the final solution is 0.94 M.
Concentration is the abundance of a constituent divided by way of the overall volume of an aggregate. several sorts of mathematical descriptions may be outstanding: mass concentration, molar concentration, variety concentration, and extent awareness.
Calculation:-
C₁ = 0.5010
V₁ = 17.5 ml = 0.0175 L
C₂ = 1.1250
V₂ = 46 ml = 0.046 L
C₁V₁ + C₂V₂ = C × total volume
0.5010 × 0.0175 + 1.1250 × 0.046 = C × 0.0635 L
C = 0.0077 + 0.052 / 0.0635 L
= 0.94 M
The concentration of a substance is the quantity of solute found in a given amount of solution. Concentrations are normally expressed in terms of molarity, defined because of the variety of moles of solute in 1 L of solution.
The Concentration of an answer is a measure of the quantity of solute that has been dissolved in a given amount of solvent or answer. A concentrated answer is one that has a rather huge quantity of dissolved solute.
Learn more about concentration here:-https://brainly.com/question/26255204
#SPJ4
Which elements are gases at STP
Answers
What’s the concentration of a sample of wine that contains 15 mL of ethyl alcohol in 200 mL of wine?
Answers
The concentration of a sample of wine that contains 15 mL of ethyl alcohol in 200 mL of wine is 7.5 % v/v.
given that :
volume of solute = 15 mL
volume of solution = 200 mL
percent volume (v/v)% = (volume of solute/ volume of solution) × 100 %
percent volume (v/v)% = (15 mL / 200 mL ) × 100 %
percent volume (v/v)% = 0.075 × 100 %
percent volume (v/v)% = 7.5 % v/v.
Thus, The concentration of a sample of wine that contains 15 mL of ethyl alcohol in 200 mL of wine is 7.5 % v/v.
To learn more about concentration here
https://brainly.com/question/13872928
#SPJ1
Complete reaction carbon-13 + alpha = oxygen-16 + x0123456789abcdefghij edited question
Answers
This is a perfect answer
ω
[tex]\text{y = ax + b}[/tex]when a chemical reaction results in a substance feeling cold, is the substance taking heat from your hand or giving heat to your hand?
Answers
When a chemical reaction results in a substance feeling cold, it is taking heat from the hand indicating it is as endothermic reaction.
"Heat is absorbed when chemical bonds are broken and released when chemical bonds are created.
Any chemical process that takes heat from its surroundings is said to be endothermic. The reaction's activation energy comes from the energy that was absorbed. This kind of reaction is characterized by its icy sensation. This may involve absorbing heat from your fingertips or the container they are in. As a result, the container and your fingers will both feel chilly.
1. Heat energy absorption is linked to these reactions.
2. The reactants' enthalpy is higher than the products' enthalpy.
3. The reaction's enthalpy is positive.
To know more about endothermic reaction visit the link:
https://brainly.com/question/23184814?referrer=searchResults
#SPJ4
a chemist has three different acid solutions. the first acid solution contains 25 % acid, the second contains 45 % and the third contains 85 % . he wants to use all three solutions to obtain a mixture of 260 liters containing 35 % acid, using 3 times as much of the 85 % solution as the 45 % solution. how many liters of each solution should be used?
Answers
Let,X = First acid solution contains 25% acid
Y = Second acid solution contains 45% acid
Z = Third acid solution contains 85% acid
Restriction given ,Z=3Y
So, X = First acid solution contains 25% acid
Y = Second acid solution contains 45% acid
3Y = Third acid solution contains 85% acid
Account for concentration of the intended acid solution;
= 25X + 45X + (3Y×85)/260 = 35
=25[X +12Y]/260 = 35
= X+12Y = 364. ————— equation (1)
Account for volume;
=X+Y+Z = 260
=X+Y + 3Y = 260
X+ 4Y = 260 ——————equation (2)
Substrate equation (2) from (1)
We get , Y = 13
Put Y = 13 in equation (2)
We get X = 208
and From X+Y+Z = 260; Z = 65
So ,208 ml of First solution ,13 ml of second solution and 65 ml of third solution should be used.
Learn more about solution here:
https://brainly.com/question/1616939?referrer=searchResults
#SPJ4
Electrolysis is performed upon molten MgCl2. platinum electrodes are used. (a) write the cathode and anode half reactions
Answers
Electrolysis occur when an electric current is passed through a liquid or solution thereby causing the decomposition of chemicals.
For magnesium chloride, it will be heated to be able to conduct electricity. When molten, the MgCl2 will decomposes into Mg2+ and 2Cl- ions. During electrolysis, the elements are separated according to the equations;
[tex]\begin{gathered} Mg^{2+}+2e^-\rightarrow Mg(s) \\ 2Cl^-\rightarrow Cl_2(g) \end{gathered}[/tex]According to the half reactions, the Mg2+ is reduced at the cathode (-) and the Cl- is oxidized at the anode (+). Reduction at the cathode shows that magnesium gains two electrons to form a magnesium solid while the chlorine ion looses two electrons at the anode
Covalent compounds are formed when metals are bonded to nonmetals? True or Flase
Answers
Answer:
Covalent compounds are always formed between two non-metals.
Explanation:
The answer is FALSE!
Covalent compounds are always formed between two non-metals. The non-metal can not lose electrons they can only gain the electron or they can share the electron to complete its octet.
Hope this helps!
Answer:
The covalent compounds are always formed by 2non-metal elements, so the answer is false
What intermolecular force is present for Ne to form a liquid at low temperatures?
a) London Dispersion Forces
b) Hydrogen bonding
c) Dipole - Dipole forces
d) Ionic bonds
Answers
Intermolecular force is present for Ne to form a liquid at low temperatures is London dispersion forces.
The hydrogen bond is present between the Hydrogen atom to the N, O, F. The dipole - dipole interaction forces are present in between two polar molecules. ionic bonds exists between two ions, that are positively charged . one is positively charged another one is negatively charged. neon exist as diatomic gas. so, the neon is a non polar .S, neon diatomic atom is present without forming any chemical bond with another atom. so., from the given option in the ques it is clear that the force between the neon atom is present as London dispersion force.
Thus, Intermolecular force is present for Ne to form a liquid at low temperatures isa) London dispersion forces.
To learn more about London dispersion force here
https://brainly.com/question/4301021
#SPJ1
if the valence atomic orbitals of an atom are sp3 hybridized, how many unhybridized p orbitals remain in the valence shell?
Answers
It is to be noted that there are no p orbitals accessible for multiple bonding in an sp3 hybrid. The two unhybridized p orbitals are accessible for multiple bonding in sp hybridization.
What is an Unhybridized P Orbital?Bonds are formed using unhybridized p atomic orbitals. Two unhybridized p atomic orbitals from distinct atoms overlap side by side, resulting in a shared electron pair filling the area above and below the connecting line between the atoms (the internuclear axis).
An atom will contain the same amount of hybridized orbitals as electron density areas. There can be a total of four hybridized sp3 orbitals, thus take the number of regions of electron density and divide that by four to get the number of unhybridized p orbitals.
An atomic orbital is a function in atomic theory and quantum mechanics that describes the position and wave-like behavior of an electron in an atom. This formula may be used to determine the likelihood of locating any atom's electron in any given location surrounding the nucleus.
Learn more about Atomic Orbitals:
https://brainly.com/question/28240666
#SPJ1
Question 3 (1 point)
Tin lives in Atlanta and observes the moon as a waxing gibbous. Her cousin lives halfway around the world in China. Do Tin
and her cousin see the same phase of the moon at the same time?
Answers
Yes, Tin and her cousin see the same phase of the moon at the same time.
What is the moon?The Moon is described as the Earth's only natural satellite. The moon is the fifth largest satellite in the Solar System and the largest and most massive relative to its parent planet, with a diameter about one-quarter that of Earth.
Tin and her cousin see the same phase of the moon at the same time because because the phase of the Moon does not change as the Earth rotates.
There are eight moon phases and they include:
These eight phases are, in order,
new Moon, waxing crescent, first quarter, waxing gibbous, full Moon, waning gibbous, third quarter and waning crescentLearn more about the moon at: https://brainly.com/question/16911469
#SPJ1
what does the S in S8 represent (Select All that Apply)
• An atom of Sulfur
• An isotope of Sulfur
• An allotrope of Sulfur
• A collection of atoms of Sulfur
Answers
Answer:
An atom of Sulfur
Explanation:
i did a Quick search and im trying to get pass the "help a person first"
Answer:
• A collection of atoms of Sulfur
Explanation:
I'll assume the notation is written as [tex]S_{8}[/tex]. A subscript after an element symbol represent the number of individual atoms of the symbol. In this case [tex]S_{8}[/tex] is telling us we have 8 sulfur atoms that are somehow connected to each other. That is in contrast with 8S, which means we have 8 different sulfur atoms. A chemical formaula, such as [tex]CH_{4}[/tex] tells us we have a molecule containing 1 carbon atom and 4 hydrogen atoms.