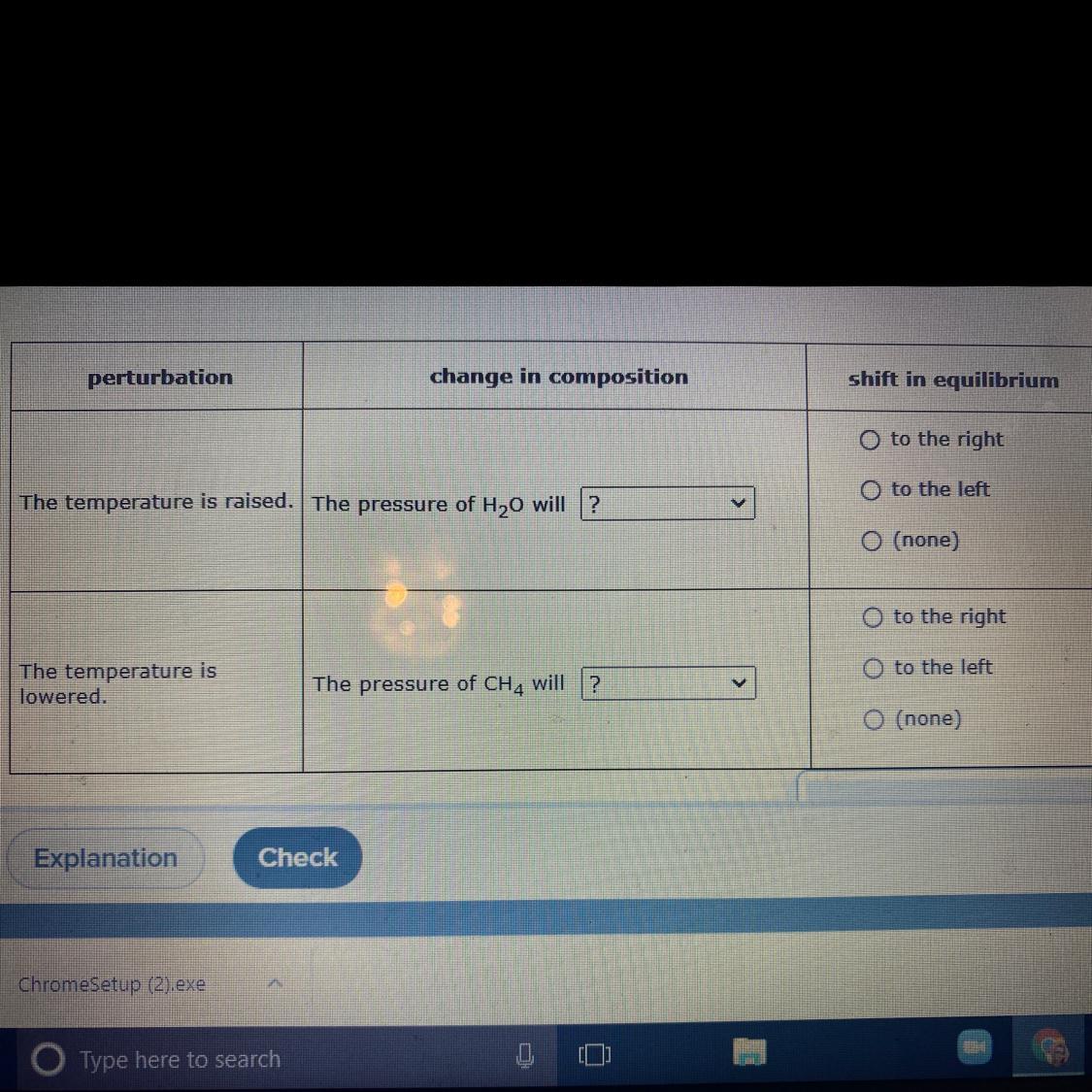
Carbon dioxide and water react to form methane oxygen like this: CO2(g)+2H2O(g)——>CH4(g)+2O2(g)The reaction is endothermic. How was the mixture of CO2, H2O, CH four and O2 has come to equilibrium in a closed reaction vessel. Predict that changed, if any, the perturbations In the table below will cause In the Composition Of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left.Perturbation: The temperature is raisedChange in composition:Pressure in the H20 will?Go up, go down, not change.Shift in equilibrium:To the right, to the left, none.Perturbation:The temperature is loweredChange in the composition:The pressure of the CH4 will?Go up, go down, not change.Shift in equilibrium:To the right, to the left, none.
Answers
ANSWER
An increased temperature will shift the equilibrium to the right and the pressure of water will be decreased.
A decreased in temperature will shift the equilibrium to the left and the pressure of the
STEP-BY-STEP EXPLANATION:
Firstly, we need to write out the balanced equation of the chemical equation.
[tex]CO_{2(g)}+2H_2O_{(g)}\rightarrow CH_{4_{((g)}}+2O_{2(g)}[/tex]What is chemical equilibrium?
Chemical equilibrium is the condition in the course of a reversible chemical reaction in which no net change in the amounts of reactant and products occurs.
According to the balanced equation, it shows that the reaction is an endothermic reaction. This means that heat was absorbed from the surroundings during the reaction.
To study the change in the equilibrium shift, we need to apply Le Chatelier's principle.
Le Chatelier's principle states that when an external constraint such as (temperature, pressure, or concentration) is imposed on a chemical system in equilibrium, the equilibrium of the system shift in order to annul or neutralize the effect of the constraints.
When the temperature of the chemical system is increased, the products side of the system will be favored. This is because an increase in temperature will lead to a faster collision between the two reactants, hence, producing more methane. Therefore, the equilibrium will shift to the left.
When the temperature is increased, the equilibrium will shift to the right.
The pressure of water will be decreased when the temperature is increased. This is because has no effect on the equilibrium system
When the temperature is lowered, the reactants side is favored and the equilibrium will shift to the left.
Looking at the balanced equation critically, the number of moles at the reactant side is equal to the number of moles at the sides of the product.
When the temperature is lowered, the pressure of methane gas will be decreased
Related Questions
A closed container contains a volume V of gas, composed of a mixture of 2 moles of O2 and 3 moles of CO2. The total pressure inside the container is 900 mm Hg. The container is at a temperature of 37⁰C? 1. Calculate PO2 and PCO2, the partial pressures of O2 and CO2 in the gas mixture. 2. Calculate the density of the gas mixture. 3. A quantity of water is introduced into the container, keeping the same quantity of gas, as before, above the water. The container is fitted with a piston, which is positioned so that the gaseous phase occupies a volume V′ and the total pressure of this gaseous phase above the water is maintained at 900 mm Hg. what is the difference between the gaseous phases in the two situations?
Answers
We have a closed container, so the number of moles remains constant.
Now, the total pressure is equal to the sum of the partial pressures. And the partial pressure of a gas will depend on its molar fraction, that is, the moles of the gas over the total moles. So the partial pressure is defined as:
[tex]P_i=\frac{n_i}{n_T}P_T[/tex]Where,
Pi is the partial pressure of the gas
ni, are the moles of the corresponding gas
nT, are the total moles
Pt is the total pressure.
1. Partial pressures of O2 and CO2
[tex]\begin{gathered} P_{O2}=\frac{2molO_2}{2molO_2+3molCO_2}\times900mmHg \\ P_{O2}=360mmHg \end{gathered}[/tex][tex]\begin{gathered} P_{CO2}=\frac{3molCO_2}{2molO_2+3molCO_2}\times900mmHg \\ P_{CO2}=540mmHg \end{gathered}[/tex]Answer: PO2=360mmHg
PCO2=540mmHg
2. Now, the density will depend of the number of moles and the volume. We can calculate the volume of the gases with the ideal gas equation that says:
[tex]\begin{gathered} PV=nRT \\ V=\frac{nRT}{P} \end{gathered}[/tex]For each gas we will have:
O2
PO2=340mmHg=0.45atm
T=37°C=310.15K
R is a constant = 0.08206 (atm.L)/(mol.K)
nO2=2mol
massO2=2mol x MolarMass = 2 mol x 31.998g/mol=63.996g
[tex]V_{O2}=\frac{2mol\times0.08206\frac{atm.L}{mol.K}\times310.15K}{0.45atm}=113.1L[/tex][tex]DensityO_2=\frac{Mass}{Volume}=\frac{63.996g}{113.1L}=0.57g/L[/tex]CO2
PCO2=540mmHg=0.71atm
T=37°C=310.15K
R is a constant = 0.08206 (atm.L)/(mol.K)
nCO2=3mol
massCO2=3mol x MolarMass = 3 mol x 44.01g/mol=132.03g
[tex]V_{CO2}=\frac{3mol\times0.08206\times\frac{atm}{mol}\times310.15K}{0.71atm}=107.5L[/tex][tex]DensityO_2=\frac{Mass}{Volume}=\frac{132.03g}{107.5L}=1.23g/L[/tex]Answer:
Density O2=0.57g/L
Density CO2 = 1.23g/L
3. In the second situation, what will happen is that the partial pressure of the gases will decrease, since due to the pressure exerted by the piston, part of the moles of gas will dissolve in the water. So in the gas phase we will have fewer moles of gas.
calculate the percent of chlorine in sodium chloride
Answers
The molar mass of sodium chloride is 58.44 g/mol.
The molar mass of chlorine is 35.45 g/mol.
To find the percent, divide the molar masses.
[tex]\frac{35.45}{58.44}\approx0.61\times100\%=61\%[/tex]Therefore, the percent of chlorine is 61%.
How many bonds are broken when performing the reaction? How many bonds are formed?(Reminder to check if the reaction is balanced)
Answers
Explanation:
We have to find the bonds that are broken and formed during the following reaction.
__ C₂H₄ + __ O₂ ---> __ CO₂ + __ H₂O
First we have to balance the reaction. To balance a combustion reaction we usually start balancing the atoms of C. We have two on the left and 1 on the right side of the equation. So the coefficient for CO₂ must be 2.
__ C₂H₄ + __ O₂ ---> 2 CO₂ + __ H₂O
Then we balance the H atoms by changing the coefficient for water. We have 4 atoms of H on the left and two on the right. The coefficient must be 2.
__ C₂H₄ + __ O₂ ---> 2 CO₂ + 2 H₂O
And finally we can balance the O atoms by changing the coefficient for O₂. We have 6 O atoms on the right side, so the coefficient for O₂ must be 3. The balanced equation is:
C₂H₄ + 3 O₂ ---> 2 CO₂ + 2 H₂O
Now to determine the bonds that have to be broken we have to pay attention to the Lewis structures of the reactants. They are:
We have to break 4 C-H bond, 1 C=C and 3 O=O bond (since the coefficient for oxygen gas is 3).
The bonds that we have to form are:
We have to form 4 C=O bonds (since the coefficient for carbon dioxide is 2), and 4 O-H bonds (since the coefficient for water gas is 2).
Answer: Four C-H, one C=C and three O=O bond need to break up. Four C=O and 4 O-H bonds need to form. (second option)
An aqueous solution (water is the solvent) of a KCl compound ( l = 2 ) boils at 102.5 degrees celcius. What is the molality (m) of the solution? Assume that the pure water boils at 100 degrees celcius.
Answers
Explanation
Given:
i = 2
boiling point of water = 100 'C
KCl boiling point = 102.5 'C
Required: Molality (m) of the solution
Solution
DT = i x Kb x m
DT = 102.5-100 = 2.5 'C
DT = i x Kb x m
2.5 = 2 x 0.512 x m
2.5 = 1.024m
molality = 2.44 m
Answer
Molality = 2.44 m
Determine the mass in grams of 3.06x10^21 atoms of arsenic.
Answers
To determine the mass of the number of atoms, the first step is to convert this number of atoms to moles using Avogadro's number:
[tex]3.06\times10^{21}atoms\cdot\frac{1molAs}{6.022\times10^{22}atoms}=0.051molAs[/tex]Convert this amount of moles to grams using the molecular weight of Arsenic:
[tex]0.051molAs\cdot\frac{74.9g}{1molAs}=3.8199g[/tex]It means that the answer is 3.8199g.
convert 12 liters to barrels
Answers
Answer:
12 liters is equal to 0.075 barrels.
Explanation:
To convert liters into barrels, it is necessary to use the following conversions:
- 1 barrel = 42 gallons
- 1 liter = 0.264 gallons
We have to write each conversion as a quotient:
[tex]12L*\frac{0.264gallons}{1L}*\frac{1barrel}{42gallons}=0.075barrels[/tex]We must write each conversion in a way that the liters cancel out, then the gallons cancel out in order to obtain the result in barrels.
So, 12 liters is equal to 0.075 barrels.
How much heat energy would be needed to raise the temperature of a 15.0 g sample of iron [Specific Heat = 0.448 J/(g°C)] from 22.0°C to 100.0°C?1. 524J2. 11J3. 249J4. 8495. 201J
Answers
Answer:
[tex]524\text{ J}[/tex]Explanation:
Here, we want to get the amount of heat energy needed
Mathematically:
[tex]Q\text{ = mc}\Delta T[/tex]where:
Q is the amount of energy that we want to calculate
m is the mass of the iron sample which is 15 g
c is the specific heat capacity which is 0.448 J/g°C
ΔT is the change in temperature which is (100-22) = 78°C
Substituting the values, we have it as:
[tex]Q\text{ = 15 }\times\text{ 0.448 }\times\text{ 78 = 524.16 J}[/tex]Consider the density data in
the table at right.
Trial #
1
The sample is thought to be
silver, density = 10.49 g/cm³.
What is the range of the data? Average
2
3
Density
(g/cm³)
10.34
10.58
10.62
10.51
Answers
10.51 (g/cm³) is the Average Density of the given data.
Average Density = 10.34+10.58+10.62+10.51÷4
Average Density=42.05/4
Average Density=10.51
Density is defined as a material substance's mass per unit volume. Density is described by the equation d = M/V, where d stands for density, M for mass, and V for volume. Grams per cubic centimeter is the unit of measurement for density.
For instance, compared to Earth's density of 5.51 grams, water has a density of 1 grams per cubic centimeter. Another way to express density is in kilograms per cubic meter (in meter-kilogram-second or SI units)
It is easy to determine the relationship between mass and volume using density. For instance, the formula for determining a body's mass is M = Vd, while the formula for determining a body's volume is V = M/d.
To know more about density visit : https://brainly.com/question/2876257
#SPJ1
The phase diagram of a substance is given below. This substance is a __ at 10 °C and 1 atm.
Answers
The phase diagram of a substance is given below. This substance is a solid at 10 °C and 1 atm.
When a material switches from one state of matter to another, a phase transition takes place. Matter exists in three basic states: liquid, solid, and gas. The many phases of a material are shown on a phase diagram using numerous variables, most frequently temperature and pressure. The figure may be used to illustrate how altering these factors impacts a substance's state of matter.
The fusion (melting or freezing) curve, which depicts the change from a liquid to a solid state. The curve that depicts the change from a gaseous to a liquid state is called the vaporization (or condensation) curve. The sublimation (or deposition) curve depicts the change from a gaseous to a solid state.
To know more about phase diagram visit : brainly.com/question/14718830
#SPJ1
If you have 4.2x10^28 Zn (zinc) atoms, how many moles of zinc do you have?
Answers
We can find the number of moles of a compound based on their number of atoms using Avogadro's number which says that there are 6.022 x 10^(23) atoms in 1 mol.
[tex]4.2\cdot10^{28}atoms\text{ Zn}\cdot\frac{1\text{ mol Zn}}{6.022\cdot10^{23}atoms\text{ Zn}}=69744.27\text{ moles Zn.}[/tex]The answer is that there are 69744.27 moles of zinc in 4.2 x 10^(28) atoms of zinc.
How many moles are there in 5.0grams of calcium bromide?
Answers
ANSWER
EXPLANATION
Given information
The mass of calcium bromide is 5.0 grams
Follow the steps below to find the mole of calcium bromide
Step1: Write the formula for calculating mole
[tex]\text{ Mole = }\frac{mass}{molar\text{ mass}}[/tex]Recall, that the molar mass of calcium bromide is 199.89 g/mol
Step 2: Substitute the given data into the formula in step 1
[tex]undefined[/tex]In the reaction below, what is the limiting reactant when 1.24 moles NH of reacts with 1.79 moles of NO?
Answers
To identify the limiting reactant, we use the coefficients of the reaction:
As you can see, we have 1.79 moles of NO, but we need 1.86 moles of NO according to the reaction. For this reason, NO is the limiting reactant since we need more than what we have.
Which of the following choices is NOT an example of a colligative property?A. Vapor pressure loweringB. Freezing point depressionC. Boiling point elevationD. Melting point acceleration
Answers
Explanation: Collagative properties are characteristics of a solution that is dependent on the ratio of solute particles to to solvent particles.
Answer: D) Melting point acceleration.
what is the electron configuration of Ni2+? How many valence electrons does this ion have?
Answers
Nickel has 28 electrons, its electron configuration is:
[tex]1s^22s^22p^63s^23p^64s^23d^8[/tex]Ni2+ has lost 2 electrons, which means that the resulting configuration would be:
[tex]1s^22s^22p^63s^23p^64s^23d^6[/tex]Nevertheless, since 4s electrons are further from the nucleus, they are lost resulting in a more stable ion, then, the actual configuration of Ni2+ is:
[tex]1s^22s^22p^63s^23p^63d^8[/tex]It means that this ion has 16 valence electrons.
How to identify Bo . compounds
Answers
Identify Bo compounds as the quantitatively by the green coloration
The presence of boron compound can be detected quantitatively by green coloration they impart to the flame of an ordinary laboratory or bunsen burner and also boron can be identified by using spectrophotometric and colorimetric method and the chemical properties of boron are more similar to carbon and silicon than element of its own group although boron is the more electron dificient
Know more about boron
https://brainly.com/question/11651796
#SPJ1
In a storage area of a hospital where the temperature has reached 55 °C, the pressure of oxygen gas in a 15.0-L steel cylinder is 965 Torr.What is the final pressure, in millimeters of mercury, when the temperature of the oxygen gas drops to 24 °C, and the volume and the amount of the gas do not change?
Answers
Answer:
[tex]873.80\text{ mmHg}[/tex]Explanation:
Here, we want to get the final pressure
From the pressure law, volume and temperature are directly proportinal
Mathematically:
[tex]\frac{P_1}{T_1}\text{ = }\frac{P_2}{T_2}[/tex]Where:
P1 is the initial pressure which is 965 torr
P2 is ?
T1 is the initial temperature which we convert to Kelvin by adding 273 K(55 + 273 = 328 k
T2 is the final temperature which is 24 + 273 = 297 K
Substituting the values, we have it that:
[tex]\begin{gathered} \frac{965}{328}\text{ = }\frac{P_2}{297} \\ \\ P_2\text{ = }\frac{297\times965}{328}\text{ = 873.80 torr} \end{gathered}[/tex]Now, we convert this to mmHg
Mathematically, 1 torr = 1 mmHg
We have the final pressure as 873.80 mmHg
2NaOH + H2504 ® Na2504 + 2H20What is the mole ratio of sodium hydroxide to water?
Answers
Given the balanced equation :
• 2,NaOH + H2S04 → Na2S04 + ,2,H20
2 : 1 :1 :2
We can see that 2 moles of NaOH produces 2 moles of H20
The ratio is 2: 2 which is the same as 1: 1 , because for every mole of NaOH , an equal amount of moles for water will be produced.An atom of which of the following elements has the greatest ability to attract electrons? Select one: a. sulfur b. nitrogen c. silicon d. chlorine
Answers
Explanation:
Electronegativity corresponds to the ability of the nucleus of an atom to attract the electrons involved in a chemical bond.
Elements belonging to the same period (same row) of the Periodic Table, we will see that electronegativity increases from left to right.
Elements belonging to the same group (same column), electronegativity increases from bottom to top.
The element among sulfur, nitrogen, silicon and chlorine that has the higher eletronegativity is chlorine.
Answer: d. chlorine
The question is in the image. If you think the question has an incorrect answer selected, then select the correct answer. Furthermore, if you can try to explain why the answer is correct and/or incorrect, I will also leave attached a text that may help you answer the question.
Answers
Compared to the normal freezing and boiling point of water, adding sugar to water will result in:
D. Lower freezing point and higher boiling point.
The chosen answer is the correct one.
This is explained as follows:
When we add sugar to water we form a solution, water molecules sorroud sugar molecules interacting with them electrostatically, this results in less interaction between water molecules and a lower amount of energy needed for them to maintain their freedom, so they will stay in liquid state at lower temperatures.
On the other hand, when we take the solution to its boiling point we will see that it is higher than that of pure water. This happens because the interaction between water and sugar molecules makes it harder for water molecules to escape the liquid phase, increasing the boiling temperature.
After swimming in the pool on a very hot day, Sarah had a glass of ice water. Over time, the water on her skin evaporated, and the ice cubes melted in the glass. Howmany states of water existed in this scenario?A. 3B. 1C. 4D. 2
Answers
3 states
Explanations:Matter can exist in three forms namely;
• Solid state
,• Liquid state
,• Gaseous state
From the given scenario, water is known to exist as ice cubes (which is the solid state). Also since the water Aon her skin evaporated the water changed to steam (gaseous state) in this case.
Also note that the ice cubes melted in the glass. This melted ice changes to liquid ,at this point. Therefore, we can conclude that the water in the scenario exists in three forms (Liquid, Solid and Gas).
Determine the percentage by mass of each element in the following compounds. (Round your answers to one decimal place.)(a)ammonia, NH3N:____%H:_____%(b)washing soda, Na2CO3Na:____%C:_____%O:_____%
Answers
To determine the mass percentage of each element in the compounds, we are going to use the following formula:
[tex]\%\text{ m/m = }\frac{mass\text{ of the element}}{mass\text{ of the compound}}*100[/tex]In the case of the last element of each compound, we are going to make the subtraction of 100% minus the addition of the other elements.
We are going to assume that we have 1 mole of every substance.
First, we have to determine the molecular weight of each substance:
a. NH3
[tex]M.W\text{ NH}_3\text{ = 14+1*3 = 17 g/mol}[/tex]If we have 1 mole of ammonia, then we have 17 g. Then, we can calculate the nitrogen mass percentage as follows:
[tex]\%\text{ m/m N = }\frac{14\text{ g }}{17\text{ g}}\text{ *100}=\text{ 82.4}\%\text{ }[/tex]Then, we make the subtraction to determine the hydrogen percentage:
[tex]\%m/m\text{ H = 100-82.4 = 17.6\%}[/tex]Then, the answer is that the nitrogen mass percentage equals 82.4% and the hydrogen one is 17.6%
b. Na2CO3
We repeat the same steps that we did with the ammonia.
[tex]M.W\text{ Na}_2CO_3\text{ = 23*2+12+16*3= 106 g/mol }[/tex][tex]\%\text{ m/m Na = }\frac{46\text{ g }}{106\text{ g}}*100=\text{ 43.4 \%}[/tex][tex]\%\text{ m/m C = }\frac{12\text{ g}}{106\text{ g }}*100\text{ = 11.3\%}[/tex][tex]\%m/m\text{ O= 100-\lparen11.3+43.4\rparen=45.3\%}[/tex]Then, the answer is that the sodium mass percentage equals 43.4%, the carbon is 11.3%, and the oxygen one is 45.3%
For the reaction NO(g) + 1/2 O2(g) -----> at 750 degrees Celsius, the equilibrium constant Kc equals
Answers
Explanation:
Given the following reaction.
NO (g) + 1/2 O₂ (g) <---> NO₂ (g)
We have to find the relationship between its Kc and Kp.
The Kp can be calculated from the Kc using the following expression.
Kp = Kc * (R*T)^(Δn)
Where Δn is the sum of the coefficients of the products minus the sum of the coefficients of the reactants. So Δn will be equal to:
Δn = Σ n of products - Σ n of reactants
Δn = 1 - (1 + 1/2)
Δn = -1/2
So we can say that:
Kp = Kc * (R*T)^(Δn)
Kp = Kc * (R*T)^(-1/2)
Kc = Kp * (R*T)^(1/2)
Answer: Kc = Kp * (R*T)^(1/2)
A physician orders a Heparin drip at 8.0 units per kg of body weight per hour via IV pump. The patient weighs 237lb. The IV is available at 25,000 units of Heparin in exactly 500 mL of IV fluid. Calculate the flow rate in mL/hr that should be set for the IV pump.
Answers
The flow rate of the IV pump should be 17.06mL/hr.
1st) We need to calculate the patient's weight knowing that 1 lb is equal to 0.45 kg:
[tex]\begin{gathered} 1\text{ lb-0.45 kg} \\ 237\text{ lb - x =}\frac{237\text{ lb}\cdot\text{0.45 kg}}{1\text{ lb}} \\ \\ x=106.65\operatorname{kg} \end{gathered}[/tex]The patient weighs 106.65 kg.
2nd) If the patient needs a drip at 8.0 units per kg of body weight, we have to calculate the units for the patient:
[tex]\begin{gathered} 1\text{ kg - 8.0 units} \\ 106.65\text{ kg - x=}\frac{106.65\text{ kg}\cdot\text{8.0 units}}{1\text{ kg }} \\ \\ x=853.2\text{units} \end{gathered}[/tex]So, the patient needs 853.2 units per hour.
3rd) To calculate the flow rate of the IV pump, we need to know the mL equivalent to 853.2 units:
[tex]\begin{gathered} 25,000\text{ units - 500mL} \\ 853.2\text{ units - x=}\frac{853.2\text{ units}\cdot\text{500mL}}{25,000\text{ units}} \\ \\ x=17.06mL \end{gathered}[/tex]So, the flow rate of the IV pump should be 17.06mL/hr.
Why does an increased temperature cause a reaction to occur faster?A. It does not. The increased temperature causes the particles to spread out and collide less.B. The increased temperature makes the molecules more loosely attached so they can switch atoms more easily.C. The increased kinetic energy causes the particles to move faster, causing more collisions.D. The increased potential energy in the particles means less energy is needed from the environment for the activation energy.
Answers
Answer
C. The increased kinetic energy causes the particles to move faster, causing more collisions.
Explanation
An increased temperature typically causes a reaction to occur faster by raising the average kinetic energy of the reactant molecules.
Therefore, a greater proportion of molecules will have the minimum energy necessary for an effective collision.
The correct answer is option C. The increased kinetic energy causes the particles to move faster, causing more collisions.
What polyatomic ions have a 3- charge?Check all that apply
Answers
Answer:
Phosphate ion only
Explanation:
Here, we want to get the polyatomic ions with a charge of -3
What we have to do here is to write the polyatomic ions and their formula
We have this as follows:
[tex]\begin{gathered} \text{Phosphate ion - PO}^{3-}_4 \\ \text{Acetate ion - }C_2H_3O^-_2 \\ Sulfate-SO^{2-}_{4^{}} \\ Hydro\times ide-OH^- \\ \\ \text{Dichromate - Cr}_2O^{2-}_7 \\ \text{Hydrogen phosphate - HPO}^{2-}_4 \end{gathered}[/tex]From the above, the polyatomic ions with a charge of -3 is the Phosphate ion only
I need to solve it and fill in the spaces
Answers
In this stoichiometry question, we have the following reaction:
4 Al + 3 N2 -> 2 Al2N3
We have:
2.5 grams of N2
We want to know how much Al we need in order to react with 2.5 grams of N2, and in order to do that, we need to find out how many moles of N2 we have in 2.5 grams, we will be using its molar mass, 28g/mol to do it:
28g = 1 mol
2.5g = x moles
28x = 2.5
x = 2.5/28
x = 0.089 moles of N2 in 2.5 grams
Now according to the molar ratio, we have 4 moles of Al for every 3 moles of N2, therefore, if we have 0.089 moles of N2:
4 Al = 3 N2
x Al = 0.089 N2
x = 0.119 moles of Al
Now we can find the mass needed, using the number of moles, 0.119 moles, and also the molar mass, 27g/mol:
27g = 1 mol
x grams = 0.119 moles of Al
x = 3.2 grams of Al is required
Answer is 3.2 grams
What is the empirical formula of P4O6?
Answers
The empirical formulas refer to the formulas where the ratios are the simplest, while the molecular formula shows the number of each type of atom.
In this case, we have a molecular formula and we just have to simplify the number to get the empirical formula. Divide each number by 2.
[tex]\begin{gathered} \frac{4}{2}=2 \\ \frac{6}{2}=3 \end{gathered}[/tex]Therefore, the empirical formula is
[tex]P_2O_3[/tex]If the pressure of a gas sample is increases 4x and the absolute temperature remains the same, by what factor does the volume of the sample change?A. 1/4B. 02C. 1/2D. 08
Answers
Answer:
The factor of change is 1/4.
Explanation:
Since in this case we have a constant temperature, the changes in the pressure will affect the volume like the Boyle's Law formula explains:
[tex]\begin{gathered} P_1*V_1=P_2*V_2 \\ \end{gathered}[/tex]To find the change of the sample volume, let's replace the initial pressure (P1) with A, and let's increase the pressure (P2) 4x:
[tex]\begin{gathered} P_1*V_1=P_2*V_2 \\ A*V_1=(A*4)*V_2 \\ \frac{A*V_1}{A*4}=V_2 \\ \frac{1}{4}V_1=V_2 \end{gathered}[/tex]So, the factor of change is 1/4.
What is the term for a hydrate with the water turned off?Answer choices:- waterless- anhydrous- dried- dehydrated
Answers
We can change a hydrate into anhydrous compound by heating it to drive off the water (dehydrate )
Correct choice is therefore anhydrous . Option 2 .
An objects heat capacity depends on which of the following?
___ Mass (quantity) of the object
___ Shape
___ Magnitude of the temperature change
___ Composition