Would greatly appreciate the help!
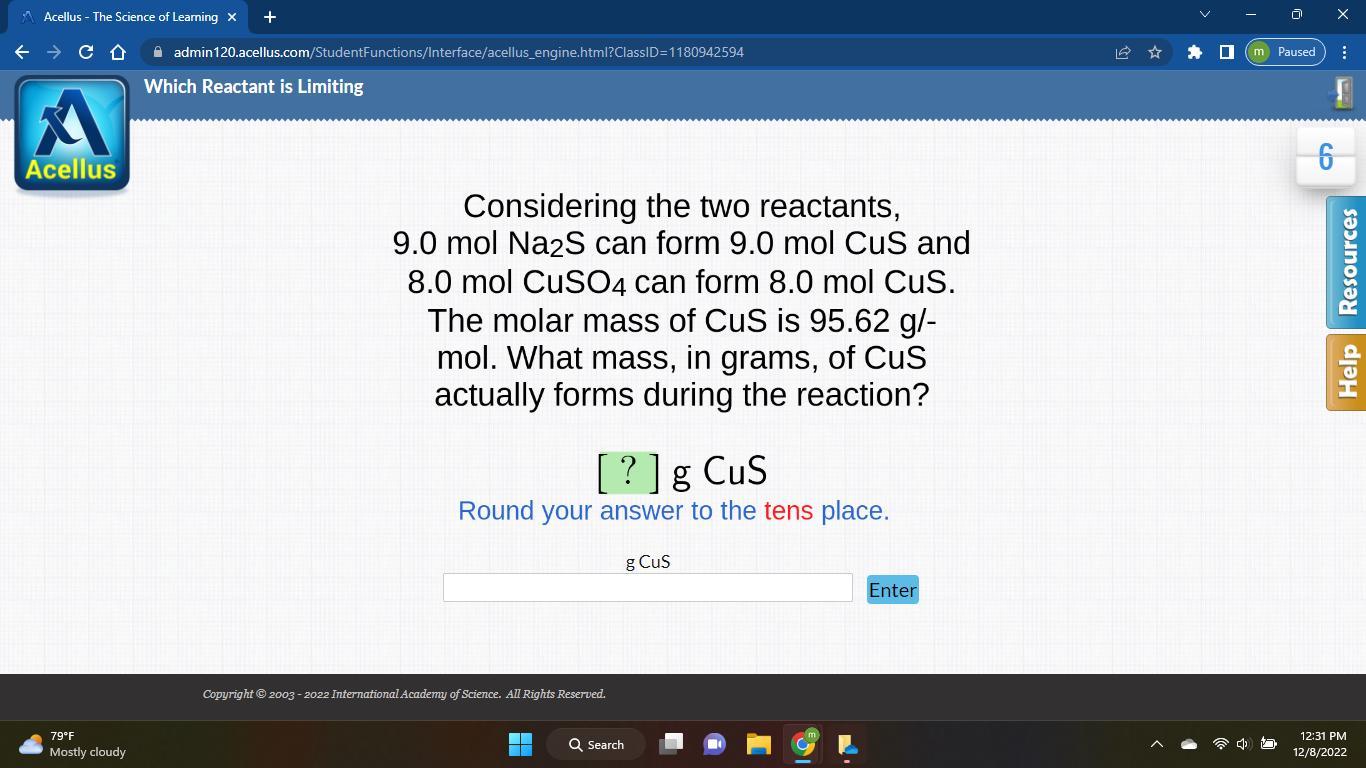
Considering the two reactants,
9.0 mol Na2S can form 9.0 mol CuS and
8.0 mol CuSO4 can form 8.0 mol CuS.
The molar mass of CuS is 95.62 g/-
mol. What mass, in grams, of CuS
actually forms during the reaction?
?]g CuS
Round your answer to the tens place.
Answers
Answer:
The total number of moles of CuS that can be formed from the given reactants is the sum of the number of moles of CuS that can be formed from each reactant, which is 9.0 mol + 8.0 mol = 17.0 mol. To find the mass of CuS that can be formed, we need to multiply the number of moles by the molar mass of CuS. The molar mass of CuS is 95.62 g/mol, so the total mass of CuS that can be formed is 17.0 mol * 95.62 g/mol = 1618.34 g. Rounded to the tens place, this is 1600 g.
Explanation:
Answer:
765.0 grams
Explanation:
Related Questions
If the temperature of a fixed amount of an ideal gas is increased, it necessarily follows that: ________
Answers
If the temperature of a fixed amount of an ideal gas is increased, it necessarily follows that: the average speed of the gas molecules will increase.
What is an ideal gas?Multiple randomly moving point particles with no interparticle interactions make create an ideal gas in a theory. The ideal gas notion is useful because it complies with the ideal gas law, has condensed equation of state, and can be studied using statistical mechanics. If, for an instance, the interaction is totally elastic or is thought of as point-like collisions, the constraint of zero interaction can frequently be disregarded.
Many real gases behave qualitatively like an ideal gas under varied temperature and pressure settings, where the gas molecules (or atoms for monatomic gases) take the place of the ideal particles.
If specific properties are preserved within appropriate bounds over a wide range of temperatures and pressures, many gases, including nitrogen, oxygen, hydrogen, noble gases, some heavier gases like carbon dioxide, and mixtures like air, can be categorized as ideal gases. At higher temperatures and lower pressures, a gas typically behaves more like an ideal gas because the potential energy from intermolecular forces is less significant compared to the kinetic energy of the particles and the size of the molecules is less significant compared to the void space between them.
To learn more about ideal gas visit:
https://brainly.com/question/28257995
#SPJ4
When hector turned 21, his friends took him out for his first drink. He ended up drinking far more than he planned that night, and he was badly hungover the next day. Hector enjoys spending time with his friends when they go out, but he doesn't want to feel sick the next day. Which strategy will help hector reduce the harmful effects of alcohol the next time he goes out?.
Answers
Hector's hangover is possibly due to the number of drinks or the type of drink he had with his friends.
Both the number of drinks and the type of drink influenced Hector's blood alcohol concentration.
To prevent the alcohol concentration from increasing in his blood, Hector basically has to dilute his drinks. On this he would base his strategies.
How to dilute drinks?One way is by adding water to the drinks and another would be choosing a drink with a low alcohol concentration, for example, beer has a lower alcohol content (5°) than whiskey (30° or more) or rum (35°).
Another strategy that Héctor can use is to dampen the alcohol concentration in the blood causing it to react with another substance, that is, the alcohol breaks down becoming another substance.
The breakdown of alcoholThere are certain pills that are sold without a prescription in any pharmacy and that serve to break down alcohol.
There are also some foods (for example, peanuts or walnuts) that, due to their magnesium content, manage to break down alcohol or at least prevent alcohol vapors from rising and going to your head.
Learn more about alcohol at brainly.com/question/14229343
#SPJ4
a mixture of carbon dioxide and oxygen gases, at a total pressure of 752 mm hg, contains 6.03 grams of carbon dioxide and 4.37 grams of oxygen. what is the partial pressure of each gas in the mixture?
Answers
The partial pressure of each gas in the mixture is 376 mm of Hg for both respectively.
Partial pressure is the force that a fuel exerts. The sum of the partial pressures in a combination of all of the gases equals the overall stress. The strain exerted with the aid of an character fuel in a mixture is known as its partial strain.
The partial pressure of an individual gas is same to the whole pressure improved with the aid of the mole fraction of that gas. because it is established completely on the wide variety of particles and no longer the identification of the gasoline, the correct fuel Equation applies just as well to combinations of gases because it does to pure gases.
Partial pressure is extremely essential in predicting the movement of gases. do not forget that gases generally tend to equalize their strain in two areas which are related. A gas will pass from a place in which its partial strain is higher to a place wherein its partial stress is lower
Calculation:-
Total pressure = 752 mm hg
carbon dioxide = 6.03 g
mole = 6.03/44
= 0.14
mass of Oxygen = 4.37
moles = 4.37/32
= 0.14
total = 0.28
partial pressure of oxygen = 0.14/0.28 × 752 mm
= 376 mm of Hg
Partial pressure of Carbondioxide = 376 mm of Hg
Learn more about partial pressure here:-https://brainly.com/question/14119417
#SPJ4
how are monomers and polymers related? group of answer choices a monomer is a small, repeating unit that makes up a polymer. a monomer is one strand of a polymer. a monomer is made when you condense a polymer through polymerization. a polymer is chemically identical to a monomer, only larger. none of these
Answers
The sole difference between a polymer and a monomer is size. A polymer is made up of tiny, repeating units called monomers. When a polymer is condensed by polymerization, a monomer is created.
The term "monomer" refers to a single atom, tiny molecule, or molecular fragment that, when joined with other monomers of the same or closely related types, produces a bigger, macromolecule known as a polymer. Through a process known as polymerization, monomers join together to form polymers through the creation of chemical bonds or supramolecular binding. Polymers are huge molecules that are created by the bonding of monomers, which are the components of a molecule. The major categories of macromolecules are known as polymers, which are macromolecules created by the union of monomers that are present in the cell such as proteins, lipids, carbohydrates, and nucleic acids
To learn more about polymers click here https://brainly.com/question/14600435
#SPJ4
if the density of your unknown liquid is 0.65 g/ml, calculate the volume in liters that 3 ml of your unknown liquid would occupy when vaporized at the barometric pressure and temperature of your boiling water bath in run 1. use the accepted molar mass of your suspected unknown.
Answers
Knowing the molar mass we can calculate the volume occupied by 3 x 0.65 g liquid in vapor phase.
When vaporized at the barometric pressure and temperature of your boiling water bath in run 1.
The boiling point of water at 1 atm. = 100 C = 100 + 273 = 373 K
standard STP temperature = 273 K
STP volume = 22.4 L
Temperature = (373/273) = 1.37
if the density of your unknown liquid is 0.65 g/ml.
density = 0.65 g/ml
Volume of 3 ml liquid when evaporated
= ((3 x 0.65)g/molar mass x 22.4 x 1.37
Substances take up space and have mass. Molecules, which make up substances. If we already know the number of moles needed then we can use the concept of molar mass to calculate how many grams of the substance are required. The molar mass is also known as molecular weight. It is the sum of the total mass in grams of all the atoms that make up a mole of a particular molecule. The unit used to measure is grams per mole.
To learn more about Molar mass please visit:
https://brainly.com/question/837939
#SPJ4
Material Density (g/cm3) Aluminum Benzene Magnesium Water Iron 2.7 0.9 1.7 1 7.8 If all of these substances were placed together in the same container, which would end up on the top?
Answers
Benzene would end up on the top, due to its low density as compared to others.
What is material density?Density (volumetric mass density or specific mass) is the mass (m) of matter per unit volume (V). Density is a standard mechanical quantity. The most common symbol for density is ρ (lowercase Greek letter rho), but the Latin letter D can also be used. Mathematically, density is defined as mass divided by volume:
ρ = m/V
For a pure substance, its density is the same number as its mass concentration. Different materials typically have different densities, which can be related to buoyancy, purity, and packaging. Osmium and iridium are the densest elements known under standard conditions of temperature and pressure.
The more dense to material get attracted by gravity and it is settle at bottom but the light one floats over it by the
To know more about density click-
https://brainly.com/question/1354972
#SPJ1
Which element is most likely to react with bromine?
Answers
Bromine reacts with many of the metals and elements. Anhydrous bromine is less reactive than wet bromine; however, dry bromine reacts vigorously with aluminium, titanium, mercury as well as alkaline earth metals and alkaline metals.
Bromine is the only liquid nonmetallic element at room temperature and one of five elements on the period table that are liquid at or close to room temperature. The pure chemical element has the physical form of a diatomic molecule, Br2.
It is a heavy, mobile, reddish-brown liquid, that evaporates easily at standard temperature and pressures in a red vapor (its colour resembles nitrogen dioxide) that has a strong disagreeable odour resembling that of chlorine. A halogen, bromine resembles chlorine chemically but is less active. It is more active than iodine, however. Bromine is slightly soluble in water, and highly soluble in carbon disulfide, aliphatic alcohols (such as methanol), and acetic acid. It bonds easily with many elements and has a strong bleaching action.
Bromine is highly reactive and is a powerful oxidizing agent in the presence of water. It reacts vigorously with amines, alkenes and phenols as well as aliphatic and aromatic hydrocarbons, ketones and acids (these are brominated by either addition or substitution reactions).
To learn more about bromine visit:
brainly.com/question/4782956
#SPJ4
select the intermolecular force that is overcome when hexane is converted from a liquid to a gas.
Answers
Electrostatic in nature, intermolecular forces result from the interaction of positively and negatively charged entities. Intermolecular interactions are the total of both attracting and repulsive elements, similar to covalent and ionic connections.
What characteristics do intermolecular forces have?
forces of attraction between molecules
Although much less than intramolecular forces of attraction, intermolecular forces play a crucial role in determining the physical characteristics of molecules, including their melting and boiling points, density, and enthalpies of fusion and vaporization.
What determines whether a reaction is intermolecular?
It can be helpful to distinguish between intramolecular and intermolecular processes. While two or more reaction sites within the same molecule are engaged in intramolecular reactions, covalency changes occur in two distinct molecules during intermolecular reactions.
To know more about intermolecular force visit;
https://brainly.com/question/9007693
#SPJ4
What is the attraction an atom has for the shared electrons in a covalent bond called?
Answers
The attraction an atom has for the shared electrons in a covalent bond called is electronegativity.
The electronegativity of the atom is the tendency of the atom to attract the share pair of electrons towards itself. the covalent bond is the bond formed by the transfer of the electrons between the atoms. if the difference in the electronegativity of the atom is higher then it will leads to the complete transfer of the electron and then the ionic bond will form and the compound is called as the ionic compound.
Thus, the electronegativity is the attraction of an atom has for the shared electrons.
To learn more electronegativity here
https://brainly.com/question/17762711
#SPJ4
when 0.434 g of sodium metal is added to an excess of hydrochloric acid, 4510 j of heat are produced. what is the enthalpy of the reaction as written?
Answers
The enthalpy of reaction is -238.62 Kj/ mol.
What is enthalpy of reaction?
Enthalpy of reaction (also known as the heat of reaction) is the amount of heat released or absorbed during a chemical reaction. It is an important physical property of a reaction, and is a measure of the reaction’s ability to do work. It is related to the change in internal energy of a reaction and can be calculated using Hess’s law. Enthalpy of reaction can be used to calculate the temperature of the reaction, the rate of reaction, and the amount of energy released or absorbed in the reaction. It is also used to determine the equilibrium constants for a reaction, and can be used to predict the stability of a reaction. Enthalpy of reaction is an important concept in thermodynamics and can be used to study the energetics of a reaction.
The enthalpy per mole of reactant is expressed in units of kj/mol.
(0.434 g of Na) × (1 mole Na / 22.990g) = 0.0189 mole
ΔH= -4.510kj /0.0189 mole = - 238.62 Kj/ mol
ΔH is negative because energy is released by the system.
For more information about enthalpy please visit: https://brainly.com/question/29888663
#SPJ4
the number of significant figures in the measurement of 45.030 mm is . question 38 options: a) three b) six c) none d) four e) five
Answers
The correct answer is Option E
e) five
What is Significant figures?
The number of significant single digits (0 through 9 inclusive) in the coefficient of an expression in scientific notation is referred to as significant figures. The amount of accuracy or assurance an engineer or scientist uses to state a quantity is indicated by the number of significant figures in a statement.
Rounding off an expression once a calculation is completed yields significant figures. The number of significant figures in the result of any computation must be equal to or less than the number of significant figures in the expression or component with the least accuracy.
Learn more about Significant Figures from given link https://brainly.com/question/30169
#SPJ4
According to molecular orbital theory, the regions of the wave function with the highest probability of finding electrons are areas with _______.
Answers
According to molecular orbital theory, regions of wave function with highest probability of finding electrons are areas with constructive interference.
An electron is a negatively charged subatomic particle that can exist either free or bound to an atom (not bound). A bound electron is one of the three primary types of particles that make up an atom, along with protons and neutrons. Protons, neutrons, and electrons combined make up the atom's nucleus. A proton's positive charge balances an electron's negative charge. When an atom has an equal number of protons and electrons, it is said to be in a neutral state. Electrons are distinct from other particles in a number of ways. They have a much lower mass, are found outside the nucleus, and exhibit both wave- and particle-like characteristics. The electron is a basic particle.
To know more about electrons visit :https://brainly.com/question/23966811
#SPJ4
rrange these compounds from fastest Sg2 reaction rate to slowest Sn2 reaction rate.
bromomethane 1-bromo-3-methylbutane 2-bromo-2-methylbutane 2-bromo-3-methylbutane
Answers
These compounds from the fastest SN2 reaction rate to the slowest SN2 reaction rate will be: Bromomethane > 1-Bromo-3-methylbutane > 2-Bromo-3-methylbutane > 2-Bromo-2-methylbutane.
The SN2 reaction is a Nucleophilic Substitution reaction in which the rate-determining step involves 2 components. Nucleophiles, typically, have a lone pair of electrons in them. They are attracted to the nucleus of an atom. In SN2 reaction an electron-rich nucleophile displaces the halogen atom bonded to the central carbon of an alkyl halide molecule. Some characteristics of SN2 reaction are: it is a one-step reaction process.
Rate of reaction depends on the concentrations of the substrate (alkyl halide) and the nucleophile.
Learn more about SN2 reaction here:
https://brainly.com/question/17031676
#SPJ4
White light passes through mercury vapor and is then analyzed using a spectrometer.
A. The observed spectrum consists of mercury spectral lines and nothing else.
B. The observed spectrum is continuous and contains mercury lines, but these lines are obscured by the white light.
C The observed spectrum is continuous without any missing lines.
D. The observed spectrum is continuous and contains dark absorption mercury lines.
E. None of the above.
Answers
The ability of spectroscopy to ascertain chemical composition pushed its development and remains one of its main applications. As well as gathering information about the universe's beginning, spectrometers are employed in astronomy to examine the chemical makeup of stars and planets.
What exactly does a spectrometer examine?
We can identify and analyze the atoms in a sample we place inside a spectrometer, which detects the wavelength and frequency of light.
When and where is a spectrometer used?
Devices that divide particles, atoms, and molecules according to their mass, velocity, or energy are examples of spectrometers. These spectrometers are employed in particle physics and chemical analysis.
To know more about the spectrometer visit;
https://brainly.com/question/4172177
#SPJ4
When white light passes through mercury vapours then The observed spectrum is continuous and contains dark absorption mercury lines.
So, Correct option is D
The capacity of spectroscopy to determine chemical composition accelerated its development and continues to be one of its primary applications. In addition to acquiring information on the origins of the cosmos, spectrometers are used in astronomy to investigate the chemical composition of stars and planets.
What does a spectrometer look for?
The atoms in a sample can be identified and analysed using a spectrometer, which measures the wavelength and frequency of light.
To learn more about spectroscopy,visit below link
https://brainly.com/question/15411034
#SPJ4
a 230-g sample of copper is heated to 100c and placed into a cup containing 340 g of water initially at 30.0c. ignore the container holding the water, assume no heat is lost or gained to the environment. 1) find the final equilibrium temperature of the copper and water
Answers
The final equilibrium temperature of the copper and water is 38.7°C. The further explanation is shown below.
To find the final equilibrium temperature of the copper and water, we need to use the equation for calculating heat transfer. The equation is as follows:
Q = mcΔT
Where Q is the heat transferred, m is the mass of the substance, c is the specific heat capacity of the substance, and ΔT is the change in temperature. In this case, we need to find the heat transferred by the copper and the heat absorbed by the water.
For the copper, the heat transferred (Q) is equal to the mass of the copper (m) times the specific heat capacity of copper (c) times the change in temperature (ΔT). The specific heat capacity of copper is 0.385 J/g°C, and the change in temperature is 100°C - 30.0°C = 70.0°C. Therefore, the heat transferred by the copper is equal to 0.385 J/g°C * 230 g * 70.0°C = 13,705 J.
For the water, the heat absorbed (Q) is equal to the mass of the water (m) times the specific heat capacity of water (c) times the change in temperature (ΔT). The specific heat capacity of water is 4.184 J/g°C, and the change in temperature is the final equilibrium temperature - the initial temperature of the water, which we don't know yet. We'll call the final equilibrium temperature T. Therefore, the heat absorbed by the water is equal to 4.184 J/g°C * 340 g * (T - 30.0°C) = 1457.6T - 44152 J.
Since the total heat transferred must be equal to the total heat absorbed, we can set the two equations equal to each other and solve for T. This gives us 13,705 J = 1457.6T - 44152 J. Solving for T, we find that the final equilibrium temperature is equal to T = (13705 J + 44152 J) / 1457.6 = 38.7°C.
Therefore, the final equilibrium temperature of the copper and water is 38.7°C.
Learn more about temperature, here https://brainly.com/question/11464844
#SPJ4
find the binding energy of carbon-12 per nucleon. the mass of a proton is 1.007276 u, of a neutron 1.008665 u, and of carbon 11.996706 u.
Answers
E of C13−BE of C12=7.5×13−7.68×12=97.5−92.16=5.34 MeV.
What practical applications exist for carbon-12?Given that it serves as the reference point for calculating the atomic masses of all other nuclides, carbon-12 is particularly significant:by definition, it has an atomic mass of 12.
Where can you find carbon-12?Numerous naturally occurring substances, including rocks like limestone, coral, as well as the shells or animals like clams, contain carbon-12.Coal and petroleum, two significant fuels, both contain carbon.
To know more about carbon-12 visit:
https://brainly.com/question/7666959
#SPJ4
if each of these ions were reduced to metal with one coulomb, which would yield the greatest mass? (a) cu2 (aq)(b) ag (aq) (c) hg2 (aq) (d) cu (aq)
Answers
The option B is correct. Ag will yield the greatest mass
Faraday proposed two electrolysis laws. The mass of an element released during electrolysis is directly proportional to the amount of electricity flowing through the electrolyte, according to the first law. Mathematically, this can be expressed as:
Mass deposited, (M) ∝ current, (I) × time, (t)
or M ∝ It.
According to the second law, the masses of different elements freed when a constant amount of power is passed through distinct electrolytes are inversely related to the chemical equivalents of the ions undergoing reaction. Making this into an equation:
″Mass deposited, M ∝ E where
E= mass of 1 mole of an ion with a charge of + 1,
So, we can see that out of all the options given Ag has the greatest value of E. So it will be the correct option.
To know more about electrolysis, please refer:
https://brainly.com/question/12994141
#SPJ4
How many dna molecules would there be after four rounds of pcr if the initial reaction mixture contained two molecules?
Answers
The number of DNA ( Deoxyribonucleic acid) molecules that are produced after 'n' number of pcr ( polymerase chain reaction) can be given by the formula: N = d x 2ⁿ
where,
'N' is the number of DNA molecules obtained after 'n' number of polymerase chain reactions.
'd' is the number of DNA molecules originally present before the start of pcr cycle.
In the given question,
d = 2;
n = 4;
Then, N = 2 x 2⁴
N = 2 x 16
N = 32;
Thus, the number of DNA molecules that are obtained after 4 rounds of pcr when 2 molecules are present before the start of the process are 32.
Learn more about DNA and PCR at:
brainly.com/question/28444229
#SPJ4
the pressure of a sample of argon gas was increased from 2.82 atm to 7.66 atm at constant temperature. if the final volume of the argon sample was 10.9 l, what was the initial volume of the argon sample? assume ideal behavior.
Answers
29.60780 (3 sig. figs.) was the initial volume of the b sample, the pressure of a sample of argon gas was increased from 2.82 atm to 7.66 atm at constant temperature. if the final volume of the argon sample was 10.9 L
At constant temperature, you are dealing with Boyle's Law (look it up and read about it).
It can be written as P1V1 = P2V2
P1 = initial pressure = 2.82 atm
V1 = initial volume = ?
P2 = final pressure = 7.66 atm
V2 = final volume = 10.9 L
P1V1 = P2V2 and solving for V1 we have...
V1 = P2V2/P1 = (7.66atm)(10.9 L) / 2.82 atm
initial volume of the argon sample V1 = 29.60780 (3 sig. figs.)
Learn more about temeperature here:
https://brainly.com/question/12035620
#SPJ4
what is the mole fraction of co in a container with a h2 mole fraction of 0.22 and an o2 mole fraction of 0.58?
Answers
Mole fraction of CO = 1 - ( 0.22 + 0.58)
=0.20
What is Mole Fraction?
The mole fraction is the product of the number of molecules of a certain component in a mixture and its total molecular weight
As a result, the mole fraction of each component added together is always equal to one.
Please be aware that mole fraction refers to a percentage of molecules, which is distinct from mass fraction because different molecules have different masses.
the proportion between the moles of one component in a solution and the moles of all the components together.
All of the components of a solution's mole fractions added together always equal one.
The mole fraction of co if the h2 mole fraction is 0.22 and the o2 mole fraction is 0.58 is 0.20, below is the solution:
Mole fraction CO + mole fraction H2 + mole fraction O2 = 1
Mole fraction CO = 1 - ( 0.22 + 0.58)=0.20
Learn more about Mole Fraction from given link
https://brainly.com/question/14783710
#SPJ4
Which of these ions is more abundant in the interior of resting neuron that in the fluid surrounding the neuron?
A. Cl-
B. Ca++
C. Na+
D. K+
Answers
K+ ions are more abundant in the interior of resting neuron that in the fluid surrounding the neuron.
An active pump can keep the larger concentration of Na+ outside the neuron because there is resistance to the passage of Na+ through the neuronal membrane. At rest, K+ concentrations are higher inside the neuron.
Which ions are more abundant outside of a resting cell?
The sodium ion concentration inside the cell is low, whereas the sodium ion concentration outside the cell is higher.
What ion contributes the most to the resting potential of neurons?
Potassium is the main ion responsible for determining the resting membrane potential. In skeletal muscle, potassium conductance makes up around 20% of the resting membrane conductance, and it makes up the majority of the resting conductance in neurons and nerve fibers.
To know more about neurons visit
brainly.com/question/29462317
#SPJ4
100 POINTS HELP PLEASEEE BRAINLIEST
Answers
Answer:
C
Explanation:
The answer is 10°c
PLEASE MARK AS BRAINLIEST
12.7 L of argon at 33°C and 735 torr are dissolved in enough water to give a final volume of 0.750 L. What is the molarity of the resulting solution 6.02 M 0.652 M 0.0644 M 0.489 M 4.95 M Con
Answers
The molarity of the gas can be obtained from the parameters in the question as 0.65 M. Option B
What is the molarity?We first have to take our minds back so that we can be able to understand what we mean by the molarity of a solution. We know that molarity has to do with the amount of the substance that can be found in the solution. In this case, we have to also know that the molarity can only be expressed in the units that we call mole per litre.
We now have to find the number of moles of the gas and this can only be obtained by the use of the ideal gas equation and that is what we are going to be able to do now in this problem.
PV = nRT
P = pressure
V = volume
n = Number of moles
R = gas constant
T = temperature
n = PV/RT
n = 0.97 * 12.7/0.082 * 306
n = 12.3/25.1
n = 0.49 moles
Molarity of the solution = Number of moles/volume
= 0.49 moles/0.750 L
= 0.65 M
Learn more about molarity:https://brainly.com/question/8732513
#SPJ1
the theoretical hvap for hexane is 28.88kj/mol. in terms of intermolecular forces, explain the difference between the heat of vaporization of water and hexane.
Answers
the theoretical hvap for hexane is 28.88kj/mol whereas of water is 2260KJ/mol, it means that intramolecular forces in hexane are weaker than that of water, thus, lesser heat is required for vaporisation.
The amount of energy required to turn a portion of a liquid substance into a gas is known as the enthalpy of vaporization, commonly referred to as the heat of vaporization or heat of evaporation. The pressure at which the transformation occurs affects the enthalpy of vaporization.
To know more about heat of vaporization, click here,
brainly.com/question/26306578
#SPJ4
rutherford found the size of the nucleus to be about. this implied a huge density. what would this density be for gold?
Answers
Rutherford found the size of the nucleus to be about 10⁻¹⁵ m, and the density for gold is 6.25 × 10²⁰ kg/m³.
Size of nucleus = 10⁻¹⁵ m
Density of gold = ?
Mass of Gold = 197 amu
Or
Mass of Gold = 197 × 1.66 × 10⁻²⁷ kg
First we will calculate the volume of the gold nucleus.
V of gold nucleus = (4 / 3) × pi × R³
Calculate the radius of the gold nucleus
Radius of gold nucleus = diameter ÷ 2
Radius of gold nucleus = 0.5 × 10⁻¹⁵ m, So
Now calculate the density
Density = 197 × 1.66 × 10⁻²⁷ ÷ 4 × pi × (0.5 × 10⁻¹⁵)³⁾³
Density = 6.25 × 10²⁰ kg/m³
The complete question is attached.
You can also learn about density from the following question:
https://brainly.com/question/15164682
#SPJ4
What is the standard cell potential for the cell Zn Zn2+?
Answers
The standard cell potential for Zn/Zn2+ couple is E°cell = 0.76 V
Zinc has an electrode cell potential of -0.76 V
Each electrode behaves anodically in relation to its local electrolyte when the circuit is open, and the voltmeter measures the sum of these two voltages, or 1.10 V. Between the two electrodes in a closed circuit, current flows. Copper functions as the galvanic cell's cathode and zinc as its anode, respectively (consuming electrons).
The zinc electrode dissolves (corrodes or oxidises), while the copper electrode takes up copper atoms from the solution (electroplating or reduction). As long as the circuit remains closed, this procedure continues.
Learn more about cell potential here:
https://brainly.com/question/17060277
#SPJ4
would it be better to use an unknown acid sample of a siz3e that should require 10 ml or one that would require 15 ml of titrant
Answers
It be better to use an unknown acid sample of a size that should require 10 ml or one that would require 15 ml of titrant.
Define titration process.
Titration is a method of chemical analysis where the amount of a sample's ingredient is determined by adding an exact known amount of a different substance to the measured sample in which the desired constituent interacts in a specific, known proportion.
It doesn't matter how much titrant is needed; what matters is how to get to the titration's end point.
Although an unknown acid sample of a size that should require 10.00 ml of titrant will be better to reduce the time of mathematically calculation only.
Therefore, it be better to use an unknown acid sample of a size that should require 10 ml or one that would require 15 ml of titrant.
To learn more about titration process from the given link.
https://brainly.com/question/27107265
#SPJ4
in the second step of glycolysis, the cyclized form of glucose (now phosphorylated) is converted to a cyclized form of fructose. what is the purpose? group of answer choices
Answers
In the second step of glycolysis, the cyclized form of glucose (now phosphorylated) is converted to a cyclized form of fructose. the purpose is the fructose is more symmetrical.
The fructose is more symmetrical. The primary goal of glycolysis is to split a 6-carbon glucose molecule into two 3-carbon pyruvate molecules. Making the molecule as symmetrical as feasible early on is a part of the method used. In contrast to cyclized glucose, which only has one external carbon, cyclized fructose can take on a variety of configurations, one of which contains four internal carbons and both the 1-C and the 6-C external to the ring. In terms of symmetry and the capacity to be divided into nearly equal 3-C units, that last form is preferable to glucose (which through a bit of processing will indeed become identical 3-C pyruvates).
To learn more about glycolysis Please click on the given link:
https://brainly.com/question/1966268
#SPJ4
a solution is prepared at that is initially in propanoic acid , a weak acid with , and in potassium propanoate . calculate the ph of the solution. round your answer to decimal places
Answers
The pH of the solution is 4.16.
What is pH?
pH is a numerical indicator of how acidic or basic aqueous or other liquid solutions are. The phrase, which is frequently used in chemistry, biology, and agronomy, converts the hydrogen ion concentration, which typically ranges between 1 and 10⁻¹⁴ gram-equivalents per liter, into numbers between 0 and 14.
Given,
Concentration of propanoic acid = 0.45 M
Concentration of potassium salt = 0.086 M
Now, Kₐ of propanoic acid = 1.3 × 10⁻⁵
∴ pKₙ = -log (1.3 × 10⁻⁵)
= 4.886
Using Henderson-Hasselbalch equation
pH = pKₐ + log [salt]/[acid]
= 4.886 + log(0.086)/(0.45)
=4.16
pH= 4.16.
To know more about pH, check out
https://brainly.com/question/172153
#SPJ4
What is a good description of bacterial reproduction? (1 point)
Responses
rapid reproduction through binary fission
slow reproduction through sexual reproduction
fast reproduction through sexual reproduction
slow reproduction through binary fission
Answers
A good description of bacterial reproduction is rapid reproduction through binary fission. That is option A.
What is binary fission?Binary fission is defined as the type of asexual reproduction whereby an organism multiplies to form new organisms through the separation of the body into two new bodies.
The process of binary fission include the following:
The organism such as bacteria, duplicates its genetic material( deoxyribonucleic acid).It then divides into two parts (cytokinesis),After each division, the new organism receive one copy of DNA. each.Under ideal conditions some bacterial species may divide every 10–15 minutes—a doubling of the population at these time intervals.
Therefore, the good description of bacterial reproduction is rapid reproduction through binary fission.
Learn more about reproduction here:
https://brainly.com/question/29762377
#SPJ1