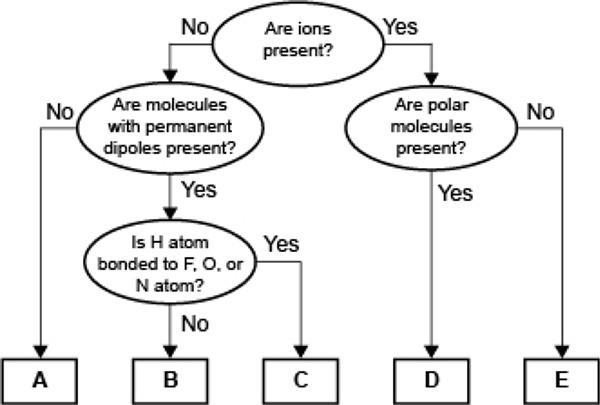
Which of the following correctly identifies the intermolecular force represented by A and compares its strength relative to the intermolecular force represented by B? A represents London dispersion forces, which are weaker than the force represented by B. A represents hydrogen bonding, which is weaker than the force represented by B. A represents London dispersion forces, which are stronger than the force represented by B. A represents hydrogen bonding, which is stronger than the force represented by B.
Answers
Answer
A represents London dispersion forces, which are weaker than the force represented by B.
Explanation
London dispersion, also called van der Waals forces are caused by the distribution of electrons throughout the molecule/atom of the compound. It is the weakest intermolecular force. The London dispersion force is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles. This force is sometimes called an induced dipole-induced dipole attraction. London forces are the attractive forces that cause nonpolar substances to condense to liquids and to freeze into solids when the temperature is lowered sufficiently.
According to the given chart, the statement which correctly identifies the intermolecular force represented by A and compares its strength relative to the intermolecular force represented by B is: A represents London dispersion forces, which are weaker than the force represented by B.
Hence, the first option is the correct answer.
Answer:A represents London dispersion force which are weaker than the force represented by B
Explanation:
Related Questions
A 4.00-L vessel contained 1.53×10-1 mol of gaseous PCl3, 1.43×10-1 mol of gaseous PCl5, and 1.20×10-1 mol of Cl2 gas at equilibrium at 245°C.
Calculate the value of Keq (expressed in terms of the molar concentrations) for the reaction:
PCl3(g) + Cl2(g) ⇌ PCl5(g)
Answers
The equilibrium constant Keq is the ratio of product of concentration of products in a reaction to that reactants. In the given reaction the equilibrium constant is 31.5.
What is equilibrium constant?The equilibrium constant in a reaction is the ratio of product of molar concentrations of products to the product of molar concentration of the reactants in the reaction.
In the given reaction, the product is PCl₅ and reactants are Cl₂ and PCl₃. Thus the expression for Keq is written as:
[tex]\rm Keq = \frac{[PCl_{5}]}{[PCl_{3}][Cl_{2}]}[/tex]
Apply the number of moles and volume of all the reactants and products in this expression.
Keq = (0.143/4 L ) [(0.12/4L) (0.15/ 4L)]
= 32.5
Hence, the equilibrium constant Keq is 32.5.
To find more on equilibrium constant, refer here:
https://brainly.com/question/10038290
#SPJ1
QUESTION 16What is the empirical formula that corresponds to a compound that contains 57.69% C, 11.54% H, and 30.77% O by mass?O C5H1202O C2.5H6OC3H1202O C58H12031С6HO3
Answers
Answer
[tex]C_5H_{12}O_2[/tex]Explanation
Given:
57.69% C, 11.54% H, and 30.77% O by mass,
Meaning:
Mass of carbon = 0.5769 g
Mass of Hydrogen = 0.1154 g
Mass of Oxygen = 0.3077 g
We know:
Molar masses of:-
C = 12.0107 g/mol
H = 1.00794 g/mol
O = 15.999 g/mol
Required: Empirical formula
Solution:
Step 1: Calculate the number of moles of the 3 atoms
C : 0.5769 g/12.0107 g/mol = 0.04826 mol
H : 0.1154 g/1.00794 g/mol = 0.1145 mol
O : 0.3077 g/15.999 g/mol = 0.0192
Step 2: Divide the moles by the lowest number of moles
In this case divide by 0.0192
C: 2.5
H: 6
O:1
Step 3: multiply by 2 to remove a decimal
C: 5
H: 12
O:2
Therefore the answer is: C5H12O2
.calculate amount of heat required to rais the temperature of 50 g og copper by 70 K? (specific heat of copper is 0.385 J/g.K.
Answers
To determine the heat required (Q) to increase the temperature of a substance we can apply the following equation:
[tex]Q=mCp\Delta T[/tex]where,
m is the mass of the substance, 50g
Cp is the specific heat of the substance, 0.386J/g.K
deltaT is the temperature difference, 70K
Now, we have the values we need to calculate the heat required, we replace the known data into the equation:
[tex]Q=50g\times0.385\frac{J}{g.K}\times70K=1347.5J[/tex]The heat required to raise the temperature of 50 g of copper by 70 K will be 1347.5J
A sample of 63.6 grams of copper completely reacted with oxygen to form 71.6 grams of a copper oxide product. How many grams of oxygen must have reacted?
Answers
Let us first illustrate the reaction of Copper and Oxygen
• 2Cu +O2 → 2CuO
ratioon > 2 : 1
step 1 : determine moles of Cu and O2 .
(i) Moles Cu = mass Cu/Molar Mass Cu
= 63.6 g/ 63.546g/mol
=1.001 mol
(ii) moles O2 = mass Oxygen/Molar Mass Oxygen
= 71.6/32
= 2.238mol
Step 2 :
Experimental moles of Cu : O2
1.001moles : 2.238moles
take note that excess reactant is O2 and limiting reactant is Cu
Therefore moles of Oxygen that reacted = 1/2 moles of Cu
= 1/2 * 1.001 = 0.5moles
Mass of Oxygen reacted =0.5* 32 (oxygen Molar mass)
=16.01g
This means that 16.01 grams of Oxygen reacted , this value is not in the options provi
Solutions are:Group of answer choicesPure substancesHomogeneous mixturesHeterogeneous mixturesAlways water-based
Answers
ANSWER
Solutions are homogenous mixtures
EXPLANATION
A solution is a mixture that contains two or more substances in the right proportions.
Solution = solute + solvent
The solution is always a homogenous mixture because the substances are mixed in the right proportion
Hence, solutions are homogenous mixtures
The number of electrons in the L orbital of an element with atomic number 10 is?
Answers
The electronic distribution of the element is going to tell us how the electrons are distributed all across the atom.
There are some concepts to check:
Energy levels: They are numbered from 1 to 7 and correspond to the element period.
In each energy level, there are 4 sub energy levels: s, p, d, and f.
The quantic number l refers to the angular position of the angle that forms the element. The number of electrons present on this orbital is equal to the addition of the last energy level, in this case, 2.
The electronic configuration for this element is:
[tex]1s^2\text{ 2s}^2\text{ 2p}^6[/tex]Then, the number of electrons in the last energy level is 8.
What is the molarity of a solution that contains 98 g of H2SO4 in 0.05 L of solution?
Answers
Answer
20 mol/L
Explanation
Given:
Mass of H₂SO₄ = 98 g
Volume = 0.05 L
From the Periodic Table; Atomic mass (H = 1.0 g, O = 16.0 g, S = 32.0 g)
What to find:
Molarity of the solution.
Step-by-step solution
Step 1: Convert the given mass of 98 g to mole.
The formula to determine the mole is given by:
[tex]\text{mole }=\frac{Mass}{Molar\text{ mass}}[/tex]Molar mass of H₂SO₄ = (2 x 1.0 g) + 32.0 g + (4 x 16.0 g) = 98 g/mol
So mole is
[tex]\text{Mole }=\frac{98\text{ g}}{98\text{ g/mol}}=1.0\text{ mol}[/tex]Step 2: Determine the molarity
The molarity formula is given by:
[tex]\text{Molarity }=\frac{Mole}{Volume\text{ in L}}=\frac{1.0\text{ mol}}{0.05\text{ L}}=20\text{ mol/L}[/tex]Ascorbic acid (vitamin C, C6H8O6) is a diprotic acid (Ka1 = 8.0×10^–5 and Ka2 = 1.6×10^–12). What is the pH of a 0.240 M solution of ascorbic acid?
Answers
Explanation:
We are given: Ka1 = 8.0*10^-5
: Ka2 = 1.6*10^-12
: concentration = 0.240M
The value of Ka1>>Ka2, therefore Ka2 will not be considered in this calculations.
We then determine the concentration of H+ at equilibrium.
Let [C6H6O6-] = x, [H+] = x
[tex][/tex]
What is the oxidation state of the nitrogen inNaNO2?1+2+3+4+
Answers
ANSWER
The oxidation state of nitrogen in NaNO2 is +3
EXPLANATION
Given information
[tex]\text{ Chemical formula }\rightarrow\text{ NaNO}_2[/tex]To find the oxidation state of nitrogen in NaNO2, follow the steps below
Step 1: Write NaNO2 in the ionic form
[tex]\text{ NaNO}_2\text{ }\rightarrow\text{ Na}^+\text{ + N + O}_2^{2-}[/tex]From the above reaction, you will see that the oxidation number of sodium is +1, and the oxidation number of oxygen is -2.
Step 2: Let the oxidation state of nitrogen be x
Add the oxidation numbers together and equate all to zero
[tex]\begin{gathered} +1\text{ + x + 2\lparen-2\rparen = 0} \\ +1\text{ + x + \lparen-4\rparen = 0} \\ +1\text{ + x - 4 = 0} \\ +1\text{ - 4 + x = 0} \\ -3\text{ + x = 0} \\ Add\text{ 3 to the both sides} \\ -3\text{ + 3 + x = 0 + 3} \\ x\text{ = 3} \end{gathered}[/tex]Hence, the oxidation state of nitrogen in NaNO2 is +3
Which phrase defines activation energy? (1 point)O energy input needed to break bonds of reactantsO energy stored in chemical bondsO energy output when product bonds are formedO energy required to break a chemical bond
Answers
The phrase tha best describes activation energy is energy input needed to break bonds of reactants.
Answer:
energy input needed to break bonds of reactants
Explanation:
Just took the quick check
Need help with chemistry 52.3 g of metal at a temperature of 126.2°C are placed in 71.2 g of water which abs an into Al temperature of 24.5°C. The final temperature of the metal and water is 35.5°C. What is the specific heat of the metal in units of J/g°C? Use 4.184 J/g°C for this problem?
Answers
Answer:
The specific heat of the metal is 0.69 J/g°C.
Explanation:
The given information from the exercise is:
• Metal,:
- Mass (massmetal): 52.3g
- Initial temperature (Tinitialmetal): 126.2°C
- Final temperature (Tfinalmetal): 35.5°C
• Water,:
- Mass (masswater):71.2g
- Initial temperature (Tinitialwater): 24.5°C
- Final temperature (Tfinalwater): 35.5°C
1st) It is necesary to write the Heat formula for both materials, the metal and water. In the case of water, we can solve the formula, because we have all the nedeed information:
• Metal,:
[tex]\begin{gathered} Q_m=m_{metal}*c_{metal}*(T_{fmetal}-T_{imetal}) \\ Q_m=52.3g*c_{metal}*(35.5_\degree C-126.2\degree C) \\ Q_m=-4,743.61g\degree C*c_{metal} \\ \end{gathered}[/tex]Water:
[tex]\begin{gathered} Q_w=m_{water}*c_{water}*(T_{fwater}-T_{\imaginaryI water}) \\ Q_w=71.2g*4.184\frac{J}{g\degree C}*(35.5\degree C-24.5\degree C) \\ Q_w=3,276.9J \end{gathered}[/tex]So, the heat absorbed by the water is 3,276.9 J.
2nd) In the end both materials reach the equilibrium temperature, because the heat released by the piece of metal is absorbed by the water, this is represented as:
[tex]-Q_m=Q_w[/tex]Finally, we have to replace the heat of the metal equation (Qm) and the heat of water (Qw) to calculate the specific heat of the metal:
[tex]\begin{gathered} -Q_{m}=Q_{w} \\ -(-4,743.61g)\degree C*c_{metal}=3,276.9J \\ 4,743.61g\degree C*c_{metal}=3,276.9J \\ c_{metal}=\frac{3,276.9J}{4,743.61g\degree C} \\ c_{metal}=0.69\frac{J}{g\degree C} \end{gathered}[/tex]So, the specific heat of the metal is 0.69 J/g°C.
H2N-(CH2)6 -NH2The compound above is one of the monomers of (A)Perupex(B)terylase(C)nylon(D)Urea
Answers
Answer
(C) nylon
Explanation
H2N-(CH2)6 -NH2 is hexamethylene diamine. The condensation reaction of hexamethylenediamine and adipic acid will produce a polymer, nylon.
Therefore, H2N-(CH2)6 -NH2 is one of the monomers of nylon
The correctanswer is (C)nylon
A gas at 100.0 kPa and 39.1°C fills a flexible container with an initial volume of 2.66 L. If the temperature is raised to 62.2°C and the pressure increased to 544.0 kPa, what is the new volume in Liters?0.5250.6300.4100.462
Answers
We have a gas that changes its volume, pressure and temperature. We will assume that there is no entry or exit of matter from the container. Therefore the moles will remain constant.
We have two states, an initial state (1) and a final state (2) for the gas. If we assume that the gas behaves as an ideal gas we can apply the following equation:
[tex]PV=nRT[/tex]Where,
P is the pressure of the gas, in atm
V is the volume of the gas, in liters
n is the number of moles of the gas
T is the temperature of the gas, in Kelvin
R is a constant, 0.08206 atm.L/mol.K
For each state we will have that the equation will be:
Initial state (1)
[tex]\begin{gathered} P_1V_1=nRT_1 \\ nR=\frac{P_1V_1}{T_1} \end{gathered}[/tex]P1 = 100.0kPa = 0.987atm
V1 = 2.66L
T1 = 39.1°C = 312.25K
Final state (2)
[tex]\begin{gathered} P_2V_2=nRT_2 \\ nR=\frac{P_2V_2}{T_2} \end{gathered}[/tex]P2 = 544.0kPa = 5.369atm
T2= 62.2°C = 335.35K
V2 = Unknown
Since R and n are constant and equal in both states of the gas, we can equate the equations and we have:
[tex]\frac{P_1V_1}{T_1}=\frac{P_2V_2}{T_2}[/tex]We clear V2 and replace the known data:
[tex]V_2=\frac{P_1V_1}{T_1}\times\frac{T_2}{P_2}[/tex][tex]\begin{gathered} V_2=\frac{0.987atm\times2.66L}{312.25K}\times\frac{335.35K}{5.369atm} \\ V_2=0.525L \end{gathered}[/tex]Answer: The new volume in liters will be 0.525
Balance these chemical equations. (Use the lowest possible whole number coefficients.)-K + Br2 → KBr
Answers
Chemistry => Chemical Reactions => Balancing equations
We have a synthesis reaction, two compounds are linked to form one.
To balance the equation we must start by counting the atoms of each element on both sides of the reaction.
We have 2 atoms of Br on the reactants and 1 atom of Br on the products. We write coefficient 2 on KBr to have the same number of Br atoms.
[tex]K+Br_2\rightarrow2KBr[/tex]Now, we continue with the K atom. We write coefficient 2 on K molecule in order to have the same number of K atoms. We will have:
[tex]2K+Br_2\rightarrow2KBr[/tex]Now, the equation is balanced.
Answer: 2K + Br2 → 2KBr
What is the correct formula that wouldresult from the combination of the two ionic species?
Answers
In order to find which ionic compound will be made from these two ions, we need to match their charges:
NH4 has a +1 charge
N has a -3 charge
We will need 3 NH4 in order to match the -3 charge of N, therefore we will have a neutral compound:
(NH4)3N is the best option, this is Ammonium nitride
I have worked through this question but am still confused on how to figure this out
Answers
We are given two solutions of C12H22O11, where solution 1 is 20% and solution 2 is 60%.
We want to know how much of each solution we need to get 38% in 50 mL of C12H22O11.
We can use the following equation:
C1V1 = C2V2
For a 20% concentration:
V1 = C2V2/C1
= (38% x 50mL)/20%
= 95mL
For a 60% concentration:
V1 = C2V2/C1
= (38% x 50mL)/60%
= 31.6mL
So to get a 50mL of 38%, you can use the 20% solution provided, you will need to use 95 mL of 20% concentration to get 38% final concentration. And if you use the 60% concentration solution provided, you will need to use 31.6mL.
Find the change in enthalpy (ΔH) for the reactions 2 H2 (g) + O2(g) --> 2 H2O (g)
Answers
Answer:
The change in enthalpy is -808 kJ.
Explanation:
The given information from the exercise is:
C-H bonds energy: 413 kJ
O=O bonds energy: 495 kJ
C=O bonds energy: 799 kJ
H-O bonds energy: 463 kJ
Chemical reaction:
[tex]CH_4+2O_2\rightarrow CO_2+2H_2O[/tex]1st) It is necessary to calculate the energy of bonds broken:
4 x (C-H) = 4 x 413 kJ = 1652 kJ
2 x (O=O) = 2 x 495 kJ = 990 kJ
Total energy of bonds broken: 1652 kJ + 990 kJ = 2642 kJ
2nd) Now we have to calculate the energy of bonds made:
2 x (C=O) = 2 x 799 kJ = 1598 kJ
4 x (H-O) = 4 x 463 kJ = 1852 kJ
Total energy of bonds made: 1598 kJ + 1852 kJ = 3450 kJ
3rd) Finally, we can calculate the enthalpy for the reaction subtracting the Total energy of bonds broken minus the Total energy of bonds made:
Change in enthalpy = Total energy of bonds broken - Total energy of bonds made
Change in enthalpy = 2642 kJ - 3450 kJ
Change in enthalpy = -808 kJ
So, the change in enthalpy is -808 kJ.
if you have 3.2 moles of copper how many moles of sodium iodide are required Nal+Cu=Cul2+Na
Answers
Step 1 - "reading" the chemical equation
The given chemical equation is:
[tex]\text{NaI}_{(s)}+Cu_{(s)}\to\text{CuI}_{2(s)}+Na_{(s)}[/tex]The proportion in moles for any chemical equation is given by the "bigger" numbers, those that come before the formula of the substances. We can use them to "read" what the equation is telling us:
1 mole of NaI react with 1 mole of Cu thus producing 1 mole of CuI2 and 1 mole of Na
Since the exercise has specifically asked us about the proportion between sodium iodide (NaI) and copper (Cu), we can simplify the statement to:
1 mole of NaI react with 1 mole of Cu
Therefore, we always need the same quantitie, in moles, of NaI and Cu for this reaction to occur completely.
Step 2 - Discovering how many moles of sodium iodide are required
Since the proportion between NaI and Cu, as we saw, is 1:1 in moles, whenever we use, let's say, x moles of Cu, we will need x moles of sodium iodide as well.
Therefore, the amount needed of sodium iodide would be exactly 3.2 moles.
For a 4s orbital ,what are the possible values of n, l and ml.
Answers
In order to know the values of the quantum numbers n, l and ml for al s4 orbital we go to a quantum table:
And as we can see to de 4s orbital correspond only the values:
n=4
l=0
ml=0
) What is the most notable difference between particles in the solid phase and the liquid phase?
Answers
Answer: Particles in the solid phase are tightly compact and they vibrate, while particles in the liquid phase move freely, randomly, and with more room to move
Explanation:
4. __H2SO4 + __Cr(OH)3 --> __Cr2(SO4)3 + __H2OYou have 4 moles of H2SO4. How many moles of H2O are produced?
Answers
The number of moles of H2O produced when 4 moles of H2SO4 are there is 8 moles of H2O
The reaction is __H2SO4 + __Cr(OH)3 --> __Cr2(SO4)3 + __H2O. The reaction is as follows;
2Cr(OH)3 + 3H2SO4 → Cr2(SO4)3 + 6H2O
3 moles of H2SO4 produces 6 moles of H2O
1 mole of H2SO4 produces 2 moles of H2O
4 moles of H2SO4 produces 8 moles of H2O
The unitary method is the method used to find the value of a single unit using the value.The number of moles is the given mass by molecular mass.n=m/Mn,m,M is the number of moles, given mass, molecular mass respectivelyTo learn more about moles visit:
brainly.com/question/26416088
#SPJ9
Part IYou blow up a balloon with your own breath and then put it in a freezer. Tell me about what happens to the size of it. Make sure to use the KMT (kinetic molecular theory) to support your answer. Part IITake the same balloon we filled up with our breath. Tell me what would happen if we put it outside and let it sit in the sun for a bit. Again, support your answer using the KMT.In both part I and part II we are going to be under the impression that no air can escape from the balloon.View keyboard shortcuts
Answers
EXPLANATION
When a balloon is blown up with the human breath, the balloon will increase in its size because there is air inside the balloon. When put in a freezer the size of the balloon will shrink, and this would reduce the size of the balloon.
Recall that, the balloon contains air, and when put in the freezer the temperature will reduce.
According to kinetic molecular theory (KMT), the average kinetic energy is a function of the temperature.
So, when the balloon is put inside the freezer, the temperature of the gas will decrease thereby decreasing the average kinetic energy. This happens because the gas molecules move more slowly and there are fewer collisions of the gas molecules with the inside wall of the balloon
When 50.0 mL of 1.0 mol/L hydrochloric acid is neutralized completely by 75.0 mL of 1.0 mol/L sodiumhydroxide in a coffee-cup calorimeter, the temperature of the total solution changes from 20.2℃to25.6℃. Determine the quantity of energy transferred, q, and state whether the reaction wasendothermic or exothermic
Answers
In this question, we have a neutralization reaction occurring and giving a value of heat, the informations we have are:
HCl = 50.0 mL and 1.0 M
NaOH = 75.0 mL and 1.0 M
ΔT = 20.2 - 25.6 = -5.4°C
We will be using the calorimetry formula in this situation, adding the values of the quantity of both reactants and using the specific heat capacity of water, c = 4.19J, the formula is:
Q = mcΔT
We have:
m = 50+75
c = 4.19J
ΔT = - 5.4
Now we add these values into the formula:
Q = 125 * 4.19 * - 5.4
Q = -2.83 kJ, this is the overall enthalpy, or energy transferred, and the minus sign in front of the number indicates that this is an exothermic reaction.
Match the reaction to the type that best describes it.NaBr + KF → NaF+ KB?Combination2Cu + MnCl2 →Mn + 2Cuci?DecompositionC + 2H2 → CH4?Single replacementCO2 + O2 + c?Double replacement
Answers
Answer:
[tex]\begin{gathered} a:\text{ Double Displacement} \\ b:\text{ Single Replacement} \\ c:\text{ Combination} \\ d:\text{ Decomposition} \end{gathered}[/tex]Explanation:
Here, we want to get the type of reactions listed
We proceed to evaluate the reactions one after the other:
a) This is a double replacement reaction. It usually occurs when molecules exchange ions in the course of their reaction leading to replacement in a molecule by an ion or a group of ions in the other molecule
b) This is a single replacement reaction which in other words could be referred to as a displacement reaction. This occurs usually when a metallic solid reacts with a solution of the salt of less reactive metal
c) This is a combination reaction. It is usually useful in the laboratory and industrial synthesis of some molecules
d) This is a decomposition reaction. It occurs when a particular molecule splits into the constituent molecules that make it up
Consider an electron transition from n = 2 to n = 4 of a hydrogen atom.
What wavelength of light will the hydrogen atom absorb or emit in this electron transition?
Answers
The required formula for doing so is
Here λ= wavelength, Rh = Rhydberg constant n1 is the the no. of orbital the electron transitioned from and n2 is the no. of orbital the electron transitioned to (ignore the z^2 as the atom in itself isn't mentioned so we assume that it is the electron of a hydrogen atom, btw z means atomic number)
So input n1 = 2, n2 = 4, Rh = 10 973 731.6 m^(-1)
you'll find the value of wavelength by inversing the answer you get from this equation directly. I don't have my calculator with me rn so can't show exact values :(
The Haber Process is performed: __N₂ + __H₂__NH,If I start with 35.0 grams of N₂ and 45.0 grams of H₂, how many grams of NH, are produced? HowMuch excess is left over?
Answers
We must first balance the equation, we have two atoms of nitrogen and hydrogen in the reactants, so we must put the coefficient 2 on the side of the products to balance the equation. The balanced equation of the reaction will be:
[tex]N_2+H_2\rightarrow2NH[/tex]Now, to find the grams of NH that will be produced, we will follow these steps:
1. We find the moles of N2 and H2 by dividing the grams by the molar mass of each element.
Molar Mass N2=28.0g/mol
Molar Mass H2=2.0g/mol
2. By stoichiometry we find out which is the limiting reactant, we see that for each mole of H2 one mole of N2 reacts, therefore, the limiting reactant will be the one with the least number of moles. The other reactant will be the excess reactant
3. We find the moles of NH using the limiting reactant.
4. We find the grams of NH by multiplying the moles by the molar mass of NH.
5. We find how much of the excess reactant is left by subtracting the initial moles minus the reacting moles.
Let's proceed with the calculations
1. Moles of N2 and H2
[tex]\begin{gathered} molH_2=givengH_2\times\frac{1molH_2}{MolarMassgH_2} \\ molH_2=45.0gH_2\times\frac{1molH_2}{2.0gH_2}=22.5molH_2 \end{gathered}[/tex][tex]\begin{gathered} molN_2=givengN_2\times\frac{1molN_2}{MolarMassgN_2} \\ molN_2=35.0gN_2\times\frac{1molN_2}{28.0gN_2}=1.25molN_2 \end{gathered}[/tex]2. Limiting reactant
We see that there are 22.5 moles of H2 and 1.25 moles of N2. The ratio N2 to H2 is 1/1, therefore, the limiting reactant will be the one with fewer moles, since it will be consumed faster. Therefore, the limiting reactant is nitrogen.
3. Moles of NH
The ratio NH to N2(Limiting reactant) is 2/1, therefore the moles of NH produced will be:
[tex]\begin{gathered} molNH=givenmolN_2\times\frac{2molNH}{1molN_2} \\ molNH=1.25molN_2\times\frac{2molNH}{1molN_{2}}=2.5molNH \end{gathered}[/tex]4. Mass of NH
[tex]\begin{gathered} MassNH=givenmolNH\times\frac{MolarMass,gNH}{1molNH} \\ MassNH=2.5molNH\times\frac{286gNH}{1molNH}=15.0gNH \end{gathered}[/tex]5. Excess of H2
[tex]\begin{gathered} molH_2(LeftOver)=molH_2initial-molH_2react \\ molH_2(LeftOver)=22.5H_2-1.25molhH_2=21.25molH_2 \end{gathered}[/tex]The mass of H2 left over will be:
[tex]\begin{gathered} MassH_2=21.25molH_2\times\frac{MolarMass,gH_2}{1mol} \\ MassH_2=21.25molH_2\times\frac{2.0gH_2}{1molH_2}=42.5gH_2 \end{gathered}[/tex]Answer: 15.0 grams of NH will be produced and the excess will be 42.5g of H2
Which of the following are examples of qualitative observations? Select all that apply.The temperature is 95.2 °C.The solution is green.The liquid is odorless.The volume is 5.6 L
Answers
Explanation:
A qualitative observation is a subjective observation, we collect data through our senses.
"The temperature is 95.2 °C" we may have measured the temperature with a thermometer.
"The volume is 5.6 L" we may have used a graduated cylinder to measure to the volume.
But if the color of the solution is green or if a liquid is odorless is something subjective. We don't see in the same way and there are lots of different greens, and we smell different. Some people are more sensible to some aromas than others.
Answer: The solution is green. The liquid is odorless.
What is the frequency of light having a wavelength of 843 nm?Speed of light 3.00x10^8
Answers
Explanation:
Frequency and wavelength are inversely proportional to each other. The equation that relates them is:
f = c/λ
Where f is the frequency, λ is the wavelength and c is the speed of light.
f = ? λ = 843 nm c = 3.00 * 10^8 m/s
Before we replace these values into the formula we have to convert the wavelength from nm to m.
1 m = 1 * 10^9 nm
λ = 843 nm = 843 nm * 1 m/(1 * 10^9 nm)
λ = 8.43 *10^(-7) m
Finally we can replace the values into the equation and get the answer to our problem.
f = c/λ
f = (3.00 * 10^8 m/s)/( 8.43 *10^(-7) m)
f = 3.56 * 10^14 1/s
f = 3.56 * 10^14 Hz
Answer: the frequency of the light is 3.56 * 10^14 Hz
estion 21 of 30hat types of bonds and atoms are in the molecule shown below?HH1H-C-C=C-C-H||||HHHHOA. Single bonds, double bonds, carbon atoms, and hydrogen atomSingle bonds double bonds and only carbon atoms
Answers
According to the given picture, in the molecules there are single bonds, double bonds, carbon atoms and hydrogen atoms. It means that the correct answer is A.
What is the net ionic equation for the reaction between HCL(aq) and NaHCO3(aq)
Answers
We have to write the net ionic equation for the reaction between HCl and NaHCO₃.
The molecular equation will show us the products of this equation.
Molecular equation:
HCl (aq) + NaHCO₃ (aq) ------> NaCl (aq) + CO₂ (g) + H₂O (l)
Now we can split this compounds into their ions to get the total ionic equation:
Total ionic equation:
H+ (aq) + Cl- (aq) + Na+ (aq) + HCO3- (aq) ----> Na+ (aq) + Cl- (aq) + CO₂ (g) + H₂O (l)
And finally we can identify the spectator ions. The ones that we have on both sides, they are Na+ and Cl-. We can cancel them out.
Net ionic equation:
H+ (aq) + HCO3- (aq) ----> CO₂ (g) + H₂O (l)
Answer: H+ (aq) + HCO3- (aq) ----> CO₂ (g) + H₂O (l)