Which electron i, on average, farther from the nucleu: an electron in a 5d orbital or an electron in a 4d orbital?
Answers
According to Aufbau Principle electrons are to be filled in a pattern that is, starting from lower-energy atomic orbitals electrons will be filling into the higher-energy orbitals.
So, electrons will be filled in the 4d orbital before 5d orbital.
Also the electron in 4d orbital will lie closer to the nucleus as compared to the electron in the 5d orbital.
as the principle quantum number for the electron in 4d orbital is 4, which means the electron is present in shell 4, i.e. n=4.
Whereas, the principle quantum number for the electron in 5d orbital is 5, which means the electron is present in shell 5, i.e. n=5.
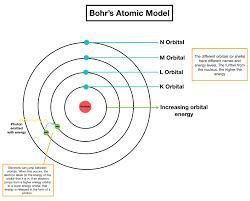
If we see Neil Bohr's atomic model,
Electrons in 4th shell will be closer to the atomic nucleus than that of electron in 5th shell.
For clarification, you can see the figure.
To know more about Quantum Number :
brainly.com/question/16977590
#SPJ4
Related Questions
a copper-zinc alloy of composition 75 wt% zn-25 wt% cu is slowly heated from room temperature. (a) at what temperature does the first liquid phase form? (b) what is the composition of this liquid phase? (c) at what temperature does complete melting of the alloy occur? (d) what is the composition of the last solid remaining prior to complete melting?
Answers
A copper-zinc alloy of composition 75 wt% zn-25 wt% cu is slowly heated from room temperature. Nickel alloy composition : 50 wt% Ni - 50 wt%. initial temperature = 1200⁰c = 2190⁰F
At What temperature the first liquid phase form?The temperature at which the first liquid phase form from the attached diagram the temperature at which the first liquid if formed is 1270⁰c ( at point 2 )
The composition of this liquid phase ( THE FIRST LIQUID ) the composition is found at point 3 wt % of Nickel = 35%, wt% of copper = 100 - 35 = 65%
The temperature at which the alloy melts completely from the attached diagram the temperature = 1320⁰c ( point 4 ).The composition of the last solid remaining prior to complete melting.
Therefore, A copper-zinc alloy of composition 75 wt% zn-25 wt% cu is slowly heated from room temperature. Nickel alloy composition : 50 wt% Ni - 50 wt%. initial temperature = 1200⁰c = 2190⁰F
Learn more about copper-zinc on:
https://brainly.com/question/29581169
#SPJ1
Is calcium chloride better for concrete than sodium chloride?
Answers
Yes, calcium chloride is better for concrete then sodium chloride because sodium chloride can damage the driveway.
Usually calcium chloride solution is used to melt the ice on the driveways and walkways.
Sodium chloride is used for this purpose as well. But sodium chloride can damage the concrete.
Calcium chloride has a very low freezing point, it form a pellets over the walkways with stops further damage to the concrete.
This thing is not possible in the case of sodium chloride. There is always a risk that there will be a greater damage to the concrete.
To know more about sodium chloride, visit,
https://brainly.com/question/24878544
#SPJ4
Two particles have the symbols Fe 54
26
2+ and Co 59
27
3+.
Which statement about these particles is correct?
Answers
The statement about cobalt and iron particle are incorrect.
What is Cobalt?Cobalt is one of the element of periodic table. It has 27 as atomic number and 59 as mass number. The electronic configuration of cobalt is 1s2 2s2 2p6 3s2 3p6 4s2 3d7. It accept three more electrons to get stable electronic configuration of the noble gas. That's why it have -3 as valancy.
What is Iron?Iron is one of the element of periodic table. It has 26 as atomic number and 56 as mass number. The electronic configuration of cobalt is 1s2 2s2 2p6 3s2 3p6 4s2 3d6. It accept four more electrons to get stable electronic configuration of the noble gas. That's why it have -4 as valancy.
Since the atomic mass of iron is incorrect which is 56 and valancy of cobalt is -3 but given valancy is +3. Therefore, the given Statments are incorrect.
Thus, we concluded that the statements about cobalt and iron particle are incorrect.
learn more about electronic configuration:
https://brainly.com/question/14283892
#SPJ1
Examine the cell below. This cell would best be described as a
Answers
Correct answer is b. Eukaryotic cell because it has nucleus and membrane bound organelles.
This image is that of an animal cell and animal cells are eukaryotic. The image shows a nucleus and membrane bound organelles.
What is the difference between eukaryotic cell and prokaryotic cell?An animal cell is cell that has nucleus, cell membrane, cytoplasm and mitochondria. These are not found in plane cells.
The main difference between prokaryotes and eukaryotes is with regards to a membrane-bound nucleus. Most specifically, eukaryotes have a membrane-bound nucleus while the prokaryotes are without it.
Other differences are:
1) No prokaryotic cell has a nucleus; every eukaryotic cell has a nucleus.
2) Prokaryotic cells have no mitochondria; nearly every eukaryotic cell has mitochondria.
3) Prokaryotic cells have no organelles enclosed in plasma membranes; every eukaryotic cell has a nucleus and organelles, each enclosed in plasma membranes.
To know more about prokaryotes and eukaryotes, click on https://brainly.com/question/2088739
#SPJ1
Which has highest negative reduction potential?
Answers
The most likely to decline is fluorine. High oxidizing substances are reduced by themselves and prefer to oxidize other substances.
Thus, fluorine has a greater potential for reduction than chlorine, bromine, and iodine. Due to low bond enthalpies and high fluorine electronegativity, there is a considerable possibility for reduction. Since reduction entails the gain of electrons, an electrode's propensity to do so is known as its reduction potential. The electrode potential is the equilibrium potential difference between the surrounding solution and the metal electrode. It can also be described as an electrode's propensity to lose or gain electrons. Positive electromotive force and spontaneous responses are characteristics of galvanic or voltaic cells.In a voltaic cell, the cathode is positive and the anode is negative. The cathode in a voltaic cell has a greater reduction potential than the anode.
To learn more about fluorine click here https://brainly.com/question/1940697
#SPJ4
What is the product of the reaction of bromoethane with each of the following nucleophiles? a. CH3CH2CH2O− b. CH3C‚C- c. (CH3)3N d. CH3CH2S-
Answers
The entire bond-forming and bond-breaking process occurs in one step during the SN2 reaction.
When switching from tertiary to secondary to primary alkyl halides, the rate of reaction for SN2 increases. The opposite trend is present for SN1. The pace of reaction for the SN2 goes from primary (fastest) > secondary >> tertiary because steric hindrance rises as we move from primary to secondary to tertiary (slowest).
The alkyl halide and nucleophile both affect the pace of the reaction. It is a second order reaction as a result.
The nucleophile attacks the Br-bearing carbon's reverse side in the subsequent reactions, replacing it.
To know more about nucleophiles, visit:
https://brainly.com/question/28325919
#SPJ4
if selective precipitation is to be achieved using nacl, what minimum concentration of nacl would be required to begin the precipitation of the ion that precipitates first.
Answers
When a solute species dissolves and precipitates at the same rates, solubility equilibria are established.
These equilibria govern a variety of technical and natural processes, from water filtration to tooth decay. To control these processes effectively, one must have a thorough understanding of the variables impacting chemical solubility. The methods and concepts of equilibrium that were previously covered in this section are applied to systems involving dissolution and precipitation.
A substance can be essentially insoluble or sparingly soluble, or it can be infinitely soluble (miscible). When added to a solvent in a quantity greater than its solubility, a solute with finite solubility can produce a saturated solution, creating a heterogeneous combination of the saturated solution and the excess, undissolved solute. For instance, the equilibrium depicted below has been reached in a saturated solution of silver chloride.
AgCl(s) ⇌ precipitation dissolution
Ag⁺(aq) + Cl⁻(aq)
At the same pace as these aqueous ions mix and precipitate to form solid AgCl, an excess of solid AgCl dissolves and dissociates in this solution to create aqueous Ag⁺ and Cl⁻ ions . The equilibrium concentration of silver chloride's dissolved ions in the solution is relatively low because silver chloride is a salt that is only sporadically soluble.
To know more about solubility product, please refer:
https://brainly.com/question/1419865
#SPJ4
a 10% aqueous naoh solution at 70 f is mixed with a 70% aqueous naoh solution at 200 f to form a solution containing 40% naoh. if the mixing is done adiabatically, what is the final temperature of the solution
Answers
The end temperature of the solution is 200 F, which is the same as the beginning temperature of the two solutions.
What exactly is adiabatic mixing?
Adiabatic mixing occurs when two chemicals are combined without exchanging heat with the surrounding environment. In other words, the mixing is done in an insulated container so that no heat is lost or acquired from the surroundings. This means that the end temperature of the combined substances will be the same as their beginning temperatures.
Because both solutions are aqueous sodium hydroxide solutions, we may assume that their heat capacities and masses are equivalent. As a result, we may equalise the mass and heat capacity terms:
C1× m1 = C2 × m2
As a result, the solution's end temperature equals the beginning temperature of the two solutions, which is 200 F.
To learn more about temperature follow the given link: https://brainly.com/question/2339046
#SPJ4
when 3.0 mol cacl2 dissolves in water, how many moles of ions are in solution? a) 1.0 mole b) 3.0 moles c) 6.0 moles d) 9.0 moles e) 12 moles
Answers
The correct answer is option C
c) 6.0 moles
What is Combination Reaction?
We look forward to the outcomes of pleasant activities like putting furniture together and mixing materials to produce something wonderful. By itself, each element is not that fascinating. But if we combine all these distinct parts, we may create something more intricate and fascinating.
Similar to this, you may mix two or more reactants to create a new product in chemical processes. This reaction falls within the category of a combination reaction, often known as a synthesis reaction.
1 mole of calcium chloride = 111g
Therefore 222g of CaCl2 is equivalent to 2 moles of CaCl2
Since 1 formula unit CaCl2 gives 3 ions,
therefore, 1 mol of CaCl2 will give 3 moles of ions 2 moles of CaCl2 would give 3×2
=6 moles of ions.
Learn more about Combination Reaction from given link
https://brainly.com/question/15092182
#SPJ4
rank the reactivity of the following alkenes toward acid-catalyzed hydration from most reactive to least reactive.
Answers
The order of the reactivity of the carbo cations as shown is; 2 >1 >4 > 3
What is the order of reactivity?We know that the order of the reactivity of the alkenes is going to so much depend on the kind of cation that is formed in the first phase of the hydration reaction.
If a stable carbocation is formed in the process then the compound that leads to such a stable carbo cation would be much more reactive. In this case, we would now look at the order of the stability of the carbo cations formed by the species so as to have an idea of the ease of reaction of the alkenes.
Learn more about carbo cations:https://brainly.com/question/14014574
#SPJ1
Water waves produced by a speed boat strike a floating inner tube. Describe the motion of the inner tube as the waves pass by.
Answers
While the waves pass by, the inner tube moves vertically. An inner tube that is floating is struck by speedboat water waves. Water waves cause energy to be transferred from a particle to another.
Without really moving the objects. The inner tube will therefore move vertically rather than horizontally as the waves pass by it. A particle is a tiny, localized object that can have various physical or chemical qualities, such as mass, density, or volume, according to the physical sciences. When the inner tube was inflated, it served as both a floating lounger and a water toy. The electrical-breakdown technique is used to remove the middle section of the outer tube, revealing the freely rotating inner tube.
Learn more about volume here
https://brainly.com/question/24189159
#SPJ4
sugar is ____ in water for the two can be mixed honogeneously to form a ___?
Answers
Answer:
sugar is Soluble in water for the two can be mixed homogeneously to form a Solution
for one of the reactions, a colored product temporarily appeared. for which reaction did this occur? what do you think this product was?
Answers
The colored product temporarily appeared is cobalt phosphate.
What is a net ionic equation?
Only those chemical species that actively contribute to a chemical reaction are listed in the net ionic equation for that reaction. In the net ion equation, mass and charge must be equal.
In an acidic solution, hydrogen peroxide oxidizes iodide ions to triiodide ions, briefly giving the solution a blue/black hue. Thiosulphate ions quickly convert these triiodide ions back to iodide ions since triiodide ions consume as quickly as they form.
6NO3- ions are spectator ions in the above process involving 6Na+. To obtain the net ionic equation, these ions are removed.
The net ionic equation :
[tex]3Co^{2+} (aq) + 2 PO_{4} ^{3-} (aq)[/tex] → [tex]CO_{3} (PO_{4} )_{2} (s)[/tex]
The inorganic substance with the formula Co3(PO4)2 is cobalt phosphate. Cobalt violet is the name of the commercially available inorganic pigment. This material's thin films act as catalysts for the oxidation of water.
Therefore, the colored product temporarily appeared is cobalt phosphate.
To learn more about net ionic equation from the given link.
https://brainly.com/question/19705645
#SPJ4
what is the heat of combustion of ethane, c2h6, in kilojoules per mole of ethane? enthalpies of formation can be found in the
Answers
The heat of combustion of ethane is -1559.7 J/mol.
The equation of the reaction is;
2C₂H₆ (g) + 7O₂ (g) ⟶ 4CO₂ (g) + 6H₂O (l)
Hence;
ΔH°reaction = [4(-393.5) + 6(-285.8)] - [2(-84.7) + 7(0)]
ΔH°reaction =[(-1574) + (-1714.8)] + 169.4
ΔH°reaction = -3119.4 J/mol
If 2 moles of C₂H₆ produces -3119.4 J/mol
1 mole of C₂H₆ produces -1559.7 J/mol
What is the heat of combustion?
The heating fee (or electricity price or calorific value) of a substance, usually a fuel or food (see meals power), is the amount of heat launched at some stage in the combustion of a unique amount of it.The calorific fee is the entire strength released as heat while a substance undergoes complete combustion with oxygen below widespread conditions.To know more about the heat of combustion, click the link given below:
https://brainly.com/question/10093007
#SPJ4
What is an example of a chemical mutation?
Answers
One type of chemical mutation is DNA replication. Biological Mutagens Some mutagens deaminate DNA nucleotides (bases) in order to remove necessary changes.
such that the DNA replication machinery is confused since these bases appear to be different chemical mutation. The modifications are subsequently permanently included in subsequent rounds of DNA replication. They refer to substances that do not combine with DNA but instead change the base-pairing characteristics and structural moieties of the nucleobases. Base modifying substances alter the specificity of the hydrogen bonds between nitrogenous bases. The process of deamination involves removing an amino group from a molecule. Deaminases are the enzymes responsible for catalyzing this process. Although it can also happen in the kidney, deamination typically occurs in the liver in the human body.
Learn more about molecules here
https://brainly.com/question/19556990
#SPJ4
The electronegativities of the period-3 elements are listed on the transparency. Calculate the
electronegativity differences for the following pairs of bonded period-3 atoms.
NA and CI
Si and CI
Mg and S
AI and P
and
Si and S
Answers
The electronegativities of the period-3 elements are on the transparency. AI and P, Si and S are period-3 elements. Therefore, option D and E are correct.
What is electronegativity ?The propensity of an atom of a certain chemical element to draw shared electrons when forming a chemical bond is known as electronegativity and is denoted by the symbol. The atomic number and the separation of the valence electrons from the charged nucleus have an impact on an atom's electronegativity.
Chlorine has a 3.0 electronegativity, compared to sodium's 0.9. Because of the rather large differential of 2.1, sodium and chlorine combine to produce an ionic compound. With hydrogen's electronegativity being 2.1 and oxygen's being 3.5, there is a 1.4 discrepancy.
Thus, option D and E are correct.
To learn more about an electronegativity, follow the link;
https://brainly.com/question/17762711
#SPJ1
calculate the final molarity of h2o2 if 5.7 ml of a 3.0% w/w h2o2 solution, which has a density of 1.0 g/ml, is added to 5.7 ml of a starch-iodide solution.
Answers
The final molarity is 0.44 M, of H₂O₂ in a given solution.
What is Molarity?Molecules per litre (mol/L) are the units used to measure molarity. It is so widely used that it has its own symbol, a capital (M). A solution with a 5 mol/L concentration is referred to as a 5 M solution or as having a 5 molar concentration.
What is the Solution?A particular kind of homogenous mixture made up of two or more substances is known as a solution. A solute is a substance that has been dissolved in a solvent, which is the other substance in the mixture.
calculation:
3.0% w/w H₂O₂ solution
100 grams of H₂O₂ contains 3.0g of H₂O₂
So, 100 mL of H₂O₂ solution contains 3.0 g of H₂O₂
Density = 1g /mL
1000 mL of H₂0₂ solution contains= ? g of H₂O₂
So, therefore,
density = 1g/ml = 3 g × 1000/100
= 30 grams
As we know that
Molarity = moles of solute/volume of solution in L
Moles = mass / molar mass
molarity = (mass/molar mass) x (1 / volume of Solution (L))
= (30g/34.0147g/mol) × (1/1L)
Molarity = 0.88 M
Now, Final molarity of H₂O₂= moles of H₂O₂ added / total volume
= 0.88 M × 5.7 mL / (5.7 + 5.7) mL
Final molarity = 0.44 M
Hence, the final molarity is 0.44 M, of H₂O₂.
To know more about Molarity, check out:
https://brainly.com/question/17138838
#SPJ4
Compounds are made up of different kinds of atoms that are chemically combined. What do compounds have in common?.
Answers
Compounds have in common that they are composed of two or more different elements chemically combined in a fixed ratio.
Compounds have two main characteristics in common: they are composed of two or more different elements chemically bonded together, and they have properties that are different from those of the individual elements. Compounds are formed when atoms of two or more elements share electrons or when atoms transfer electrons from one atom to another. Different types of elements can be combined to form different compounds. For example, oxygen and hydrogen can form water, carbon and oxygen can form carbon dioxide, and nitrogen and oxygen can form nitrous oxide. The properties of a compound depend on the type of elements it is composed of and the way the atoms are chemically bonded together. Compounds are usually more stable than individual elements, and they can be broken down into simpler substances by chemical reactions.
To know more about compounds refer to the link brainly.com/question/19458442
#SPJ4
0.24g magnesium is burnt in oxygen, the mass of the magnesium oxide is 0.40g. determine the formular of magnesium oxide and the molar mass of magnesium oxide burnt
Answers
Magnesium metal reacts with oxygen and forms magnesium oxide have the formula MgO. The molar mass of MgO is 40 g/mol.
What is magensium oxide?Magnesium oxide is an ionic compound formed by losing two electrons from the magnesium metal to the oxygen. The reaction of Mg with O is written below:
[tex]\rm 2Mg + O_{2} \rightarrow 2 MgO[/tex]
As per this reaction, 2 moles of Mg gives two moles of MgO. Given that 0.24 g of Mg gives 0.40 g of Mg.Which means one mole of Mg gives one mole of MgO.
0.24 g of Mg gives 0.40 g. Thus, one mole or 24 g of mg will give 40 g of MgO.
atomic mass of Mg = 24 g/mol
atomic mass of oxygen = 16 g/mol
molar mass of MgO = 16 + 24 = 40 g/mol.
Therefore, the molar mass of magnesium is 40 g/mol.
To find more on MgO, refer here:
https://brainly.com/question/29769894
#SPJ1
A factory has a batch of hand sanitizer with 50\%50%50, percent alcohol content. How much pure (100\%100%100, percent concentration) alcohol should they include in a 600\,\text{ml}600ml600, start text, m, l, end text bottle to make a 68\%68%68, percent mixture?.
Answers
408 ml of 100% pure alcohol must be included in a 600ml bottle to have an alcohol with 68% concentration
Formula for dilution calculation[tex]V_{1} . C_{1} = V_{2} . C_{2}[/tex]
[tex]V_{1} =[/tex] Volume of solution 1
[tex]C_{1} =[/tex] concentration of solution 1
[tex]V_{2} =[/tex] Volume of solution 2
[tex]C_{2} =[/tex] concentration of solution 2
Pure Alcohol Volume CalculationData
[tex]V_{1} =[/tex] ?
[tex]C_{1} =[/tex] 100% v/v
[tex]V_{2} =[/tex] 600 ml
[tex]C_{2} =[/tex] 68% v/v
[tex]V_{1} = V_{2} . C_{2} / V_{1}[/tex]
[tex]V_{1} =[/tex] 600 x 68 / 100
[tex]V_{1} =[/tex] 408ml
Consequently, 408 ml are poured into the 600 ml container and this volume is made up with distilled water.
This procedure is used to prepare solutions such as hand sanitizers or to reduce the alcohol content in some drinks.
Learn more about alcohol volume calculation at https://brainly.com/question/17290000
#SPJ4
For the list of alcohols, rank the alcohols in strength from weakest acid to strongest acid.F2CHOH F3COH FCH2ОН CH3OH
Answers
For the list of alcohols,the rank of alcohols in strength from weakest acid to strongest acid is F3COH , F2CHOH, FCH2ОН, CH3OH
Any substance that when dissolved in water has an acidic taste, can alter the color of some indicators (such as reddening blue litmus paper), can react with some metals (such as iron) to release hydrogen, can combine with bases to form salts, and can accelerate some chemical reactions (acid catalysis). Acids are examples of both organic compounds that belong to the carboxylic acid, sulfonic acid, and phenol groups as well as the inorganic substances known as the mineral acids, such as sulfuric, nitric, hydrochloric, and phosphoric acids. These substances contain one or more hydrogen atoms, which are released as positively charged hydrogen ions when they are in solution (see Arrhenius theory). The Brnsted-Lowry theory provides broader definitions of an acid that encompass substances that exhibit typical acidic behaviour as pure compounds or when dissolved in solvents other than water.
To know more about acids visit : https://brainly.com/question/23687757
#SPJ4
Alkaline mucus made by this gland neutralizes traces of acidic urine in the urethra prior to ejaculation.a. Trueb. False
Answers
Answer:
True hope this is the correct answer if not sorry
Explanation:
1. as the acid solution is titrated the pink color forms where the drop first enters the solution but disappears as the solution is mixed. explain why this happens.
Answers
As the acid solution is titrated the pink color forms where the drop first enters the solution but disappears as the solution is mixed. Since the titrant is being added to the solution dropwise.
Titrated, also known as titrimetry and volumetric analysis, is a common laboratory method for quantitative chemical analysis used to determine the concentration of a specified analyte (a substance to be analyzed). The reagent, often referred to as the titrant or titrator, is made from a standard solution with a defined concentration and volume. The titrant reacts with an analyte solution to determine the concentration of the analyte (also known as the titrand). The volume of titrant that interacted with the analyte is known as the titration volume. The first widespread application of volumetric analysis was in late 18th-century France. François-Antoine-Henri Descroizilles invented the first burette, which resembled a graded cylinder, in 1791 (fr). Gay-Lussac first used the words "pipette" and "burette" in an 1824 study on the standardization of indigo solutions. He also described a burette with a side arm.
To know more about Titrated please refer: https://brainly.com/question/2728613
#SPJ4
Which choice is not true of a liquid in a glass capillary with a convex meniscus?a. The liquid has strong cohesive forces.b. The liquid level will be lower inside the capillary when a capillary is inserted into a bowl of the liquid.c. The liquid will have a convex meniscus as it moves in the capillary.d. The behavior of the liquid is driven by strong interactions with the capillary glass.
Answers
The correct answer is the liquid level will be lower inside the capillary when a capillary is inserted into a bowl of the liquid.
When a liquid is placed in a glass capillary with a convex meniscus, the behavior of the liquid is driven by strong cohesive forces between the liquid molecules and strong interactions with the capillary glass. As a result, the liquid will have a convex meniscus, meaning that the surface of the liquid will be curved outward. This is due to the forces acting on the liquid in the capillary. However, when a capillary is inserted into a bowl of the liquid, the liquid level inside the capillary will be higher than the liquid level outside the capillary. This is because the cohesive forces between the liquid molecules are stronger inside the capillary, where the liquid is in contact with the walls of the capillary, than they are outside the capillary. As a result, the liquid will be pulled up the walls of the capillary, causing the liquid level inside the capillary to be higher than the liquid level outside the capillary. This is opposite to what is stated in choice b, which is why it is the correct answer.
to know more about meniscus-
https://brainly.com/question/28013459
#SPJ4
which phenomenon that goes unexplained by lewis structures is solved by applying molecular orbital theory?
Answers
The paramagnetism of the O₂ molecule. Lewis structures would show O₂ as having all paired electrons, but it exhibits paramagnetism, which requires unpaired electrons. This issue can be solved by applying molecular orbital theory.
The electron is a subatomic particle with a bad standard electric charge. Electrons belong to the primary technology of the lepton particle's own family and are typically thought to be fundamental particles because they have no recognized additives or substructure.
By using the best values for the wavelength and the scattering by a matter of tough X-rays and ?-rays, the radius of the electron is anticipated as approximately 2 × 10-10 cm.
Learn more about Electron here:-https://brainly.com/question/860094
#SPJ4
suppose you start with 247g of ice at 0 oc. calculate the amount of heat energy that must be transferred to convert the ice to steam at 100 oc.
Answers
753.25 kj is the amount of heat energy that must be transferred to convert the ice to steam at 100 oc.suppose you start with 247g of ice at 0 oc.
E = mct
Where E is the energy for a phase change, m is the mass of the object, c is the specific heat capacity and t is the change in temperature.
So ignoring the latent heats' at present we can deduce that the energy required to raise the temperature of a 250g (0.25kg) block of ice by 100 degrees Celsius is:
E = 0.25*4.19*100 = 104.75kJ
Now the latent heats' tell you the energies absorbed when the ice changes state from solid to liquid to gas. This increase in energy does not change the temperature as it is used solely to create the intermolecular bonds that give rise to a change of state.So to find the total heat energy required for the conversion of the ice to steam, we just need to add the latent heats' of fusion and vaporisation.Note that the latent heats are given per kilogram so so they need to be multiplied by the mass of the ice block; this gives:
E = 104.75 + 0.25(334 + 2.26X10^3) = 104.75 + 0.25(2594) = 753.25kJ
Learn more about energy here:
https://brainly.com/question/8630757
#SPJ4
How many grams of h2o will be formed when 39. 0 g h2 is mixed with 49. 6 g o2 and allowed to completely react to form water?.
Answers
45.784 grams of water will be formed when 39 g hydrogen is mixed with 49.6 g oxygen.
The balanced reaction for the above reaction is given as:
2 H₂ + O₂ → 2 H₂O
By the relationship between the amount of reagents and products in a chemical reaction, we can conclude that the following amounts of each compound participate in the reaction:
H₂: 2 moles
O₂: 1 mole
H₂O: 2 moles
the molar mass of the compounds are as follows:
H₂: 2 g/mole
O₂: 32 g/mole
H₂O: 18 g/mole
Then, by the reaction stoichiometry, the following amounts of reactant and product mass participate:
For H₂: 2 moles × 2 g/mole = 4 g
For O₂: 1 mole × 32 g/mole = 32 g
For H₂O: 2 moles × 18 g/mole = 36 g
The limiting reagent of the reaction is one that is consumed first in its entirety, determining the amount of product in the reaction. When the limiting reagent is finished, at that time the chemical reaction will stop.
Since we have less mass than we need to react with 39 grams of H₂, oxygen O₂ will be the limiting reagent.
Then we can apply the following rule of three: if by stoichiometry 32 grams of O₂ form 36 grams of H₂O, 49.6 grams of O₂ will produce H₂O is:
mass of H₂O = (49.6 grams of O₂ × 36 grams of H₂O) / 39 grams of O₂
mass of H₂O = 45.784 grams
Learn more about amount of water produced from the link given below.
https://brainly.com/question/20814085
#SPJ4
Write the balanced chemical equation for the reaction between nitrogen oxygen. Include physical states.
Answers
The balanced chemical equation for the reaction between nitrogen and oxygen is as:
2N₂ (g) + 2O₂ (g) —> 4NO (g)
The following is the equation that can be written to describe the relationship between nitrogen and oxygen:
N₂(g) + O₂(g) —> NO (g)
The equation that was just discussed can be balanced in the following way: On the left side, there are two atoms of oxygen, whereas, on the right side, there is only one. To achieve equilibrium, write 2 in front of NO, as seen in the following example:
N₂(g) + O₂(g) —> 2NO (g)
When we multiply the equation by 2, we get the following:
2N₂(g) + 2O₂(g) —> 4NO (g)
The equation is balanced now.
You can also learn about chemical equations from the following question:
https://brainly.com/question/28294176
#SPJ4
Air pollutants regulated by the clean air act include all of the following EXCEPT? a. carbon dioxide b. particulates c. carbon monoxide d. sulfur dioxide.
Answers
Option (a) Carbon dioxide is not an air pollutant according to the clean air act prediction.
The air we breathe contain many pollutants. Air pollution can contain a mixture of solid particles, liquid droplets and gases from a variety of sources such as industry, motor vehicles, heating appliances, and tobacco smoke. Air pollution can also be generated by natural events such as bush fires and can contain windblown dust, pollen and mold spores. The composition of air pollution can vary greatly depending on the season the weather and the types and numbers of sources.
According to the clean air act, the EPA has identified six pollutants as “criteria” air pollutants because it regulates them by developing human health-based for setting permissible levels. These six pollutants are carbon monoxide, lead, nitrogen oxides, ground-level ozone, particle pollution and sulfur oxides.
To learn more about Air pollutant please visit:
https://brainly.com/question/23196476
#SPJ4
The electronegativities of the period-3 elements are listed on the transparency. Calculate the
electronegativity differences for the following pairs of bonded period-3 atoms.
d. Si and Cl _______________________
b. Mg and S_1.31. And 2.58=
e. Si and S ________________________
c. Al and P ______________________
Answers
Here is the electronegativity difference of bonded period 3 atoms.
What is electronegativity?Electronegativity, is the symbolized as χ, it is the tendency to an atom of to a given chemical element to be attract shared electrons and when forming of a chemical bond. An atom's is electronegativity is affected by the both its atomic number .and it is
The electronegativity for period -3 atoms
Si and cl is - Electronegativity Difference is 0.7
Polarity-δ+Si−δ−C
For mg electronegativity difference is 1.3
For si and s electronegativity difference is 1.7
For AI and p the electronegativity difference is 1.6 and 2.9 respectively.
To know more about electronegativity click-
https://brainly.com/question/24977425
#SPJ1