Answers
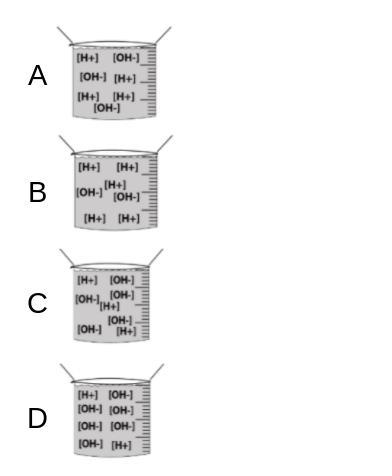
Assuming that the question is considering quantity of OH- ions as strength of base, the most basic compound will be the last one, as it has more OH- being released into the reaction, but the definition of strong base is a base that totally dissociates, but for the purpose of the question, letter D corresponds to a strong base.
Related Questions
3 Cu + 8HNO3 g 3 Cu(NO3)2 + 2 NO + 4H₂O
In the above equation how many moles of NO can be made when 75 moles of HNO3 are
consumed?
Answers
As per the given statement 0.297mol NO can be made when 75 moles of HNO3 are consumed
What is HNO3?The inorganic substance with the formula HNO3 is nitric acid. It is a mineral acid that is quite corrosive. The substance is colourless, but older samples have a tendency to have a yellow cast because of breakdown into nitrogen oxides..
'3 Cu + 8HNO3 3 Cu(NO3)2 + 2 NO + 4H₂O
Element Molar Mass
Hydrogen 1
Nitrogen 14
Copper 63.5
Oxygen 16
Referring the above table we get no of moles in HNO3
1(1.0) + 1(14) + 3(16) = 1 + 14 + 48 = 63 g/mol
75g HNO3 x 1 mol HNO3/63 g HNO3 = 1.190 mol HNO3
The coefficient of HNO3 is 8, and NO is 2
1.190 mol HNO3 x 2 mol NO/8 mol HNO3 = 0.297mol NO
Hence, 0.297mol NO can be made when 75 moles of HNO3 are consumed.
To learn more about HNO3 visit to the given link
https://brainly.com/question/26015251
#SPJ1
___ H2SO4 + __KOH → __K2SO4 +__H2 Obalance the equation
Answers
Explanation:
We have to balance this equation:
___ H₂SO₄ + __ KOH ----> __ K₂SO₄ + __ H₂O
The first step is to determine the number of atoms of each element that we have on both sides of the equation.
___ H₂SO₄ + __ KOH ----> __ K₂SO₄ + __ H₂O
K: 1 K: 2
S: 1 S: 1
H: 3 H: 2
O: 5 O: 5
We have 1 atom of K on the left side of the equation and there are 2 atoms of K on the rigth side of the equation. To balance the number of K atoms we can change the coefficient for KOH and write a 2 there.
___ H₂SO₄ + 2 KOH ----> __ K₂SO₄ + __ H₂O
K: 2 K: 2
S: 1 S: 1
H: 4 H: 2
O: 6 O: 5
We balanced the K. The S atoms are already balanced. Let's focus on H. We have 4 atoms of H on the left and 2 atoms of H on the right side of the equation. We can change the coefficient for H₂O and write a 2 there to get 4 atoms of H on the right side.
___ H₂SO₄ + 2 KOH ----> __ K₂SO₄ + 2 H₂O
K: 2 K: 2
S: 1 S: 1
H: 4 H: 4
O: 6 O: 6
We balanced the H and we also changed the number of O atoms on the right side and also balanced them. The balanced equation is:
H₂SO₄ + 2 KOH ----> K₂SO₄ + 2 H₂O
Answer: H₂SO₄ + 2 KOH ----> K₂SO₄ + 2 H₂O
37.In the compound MgO, what is the oxidation number of oxygen?Select one:a. +4b. +2c. +1d. -2
Answers
When we have to determine the oxidation number of compounds, we need to look at their specific and most common charges in their non neutral form, and Oxygen is an element that will usually present a -2 oxidation number and charge, the same way Hydrogen will almost always present a charge of +1, these fixed values for some elements is helpful for us to determine the oxidation number of other elements. For oxygen is -2, therefore letter D
Ammonia, NH3 , reacts with oxygen to form nitrogen gas and water.
4NH3(aq)+3O2(g)⟶2N2(g)+6H2O(l)
What is the percent yield of the reaction?
Answers
The percent yield of the reaction when 3.85g of Ammonia reacts with 5.78g of O2 to give out 0.750L of nitrogen is 27.43%
It is given that the mass of Ammonia is 3.85g and the mass of Oxygen is 5.78g. The yield of Nitrogen is 0.750L. To find the percent yield, we need to know the number of moles of ammonia and Oxygen.
No of moles of Oxygen = 5.78/31.99
No moles of Oxygen= 0.181 mol of O2.
No. of moles of Ammonia = 3.85/17.03
= 0.226 mol of NH₃
It can be seen that 0.226 mol of NH₃ reacts with 3/4th of 0.226 mol of O₂ which is 0.1695 mol of O₂.
But the given moles of O₂ are more than that of the required value. Therefore, NH₃ is the limiting reactant and O₂ is the excess.
Therefore,
0.226 mol of NH3 x 2 mol of N₂/ 4 mol of NH3
= 0.113 mol of N₂
Experimentally it would be calculated as
n = PV/RT
n = (1 x 0.750)/(0.0821x295)
n = 0.031 mol of N₂
Percent yield = Experimental yield/theoretical yield x 100
Percent yield= (0.031/0.113)x100
Percent yield= 27.43%
Therefore, the percent yield of N₂ is 27.43%
To know more mass percentage, click below:
https://brainly.com/question/29343293
#SPJ1
4. Calculate the pH of 0.90M HPO4?Ka HPO4^2- = 4.5 x 10-13(a) 7(b) 14(c) 12.3(d) 8.20(e) 6.20
Answers
Answer
Explanation
Given:
[H⁺] = 0.90 M
Ka HPO₄²⁻ = 4.5 x 10⁻¹³
A balloon is filled with a 0, gas and has a volume of 179mL at 2.00 atm pressure. What will its volume be if thepressure is changed to 5.50 atm?A) 29.2 mLB) 65.1 mLC) 17.9 mLD) 83.0 ml
Answers
In this case, we can use the Boyle's law to solve this problem:
[tex]P1\cdot v1=P2\cdot v2[/tex]We already know the values of P1, v1 and P2 and we have to find v2. Replace for the given values and solve for v2:
[tex]\begin{gathered} 179ml\cdot2.00atm=5.50atm\cdot v2 \\ v2=\frac{179ml\cdot2.00atm}{5.50atm} \\ v2=65.1ml \end{gathered}[/tex]The correct answer is B) 65.1ml.
Can you please help me fast
Answers
Answer:
C
Explanation:
the answe is naturally occurring
(give me brainliest )
Which of the following minerals is the most resistant to being scratched?
Hematite
Calcite
Orthoclase
Garnet
Answers
(p.s. if you can, please mark me brainliest, it would help a lot)
if 2.00 moles of aluminum nitrate are dissolved to form 2.00l of solution , the concentration of the NO3 ion will be ?
Answers
First, we write the reaction to solve this:
Al(NO₃)₃(aq) => Al³⁺(aq) + 3NO₃⁻(aq)
We need to calculate the concentration of the salt:
Molarity = moles of Al(NO₃)₃ / Volume of solution (L)
Molarity = 2.00 moles / 2.00 L = 1.00 mol/L
The salt is completely disociated, so the concentration of NO3- ion is:
Concentration NO3- = 3 x Concentration of the salt = 3 x 1.00 mol/L = 3.00 mol/L
Answer: 3.00 mol/L
What common similarity is possessed by all electrolytes, other than being water soluble?
Answers
INFORMATION:
Knowing that all electrolytes are water soluble, we must determine which other common similarity is possessed by all electrolytes
STEP BY STEP EXPLANATION:
To determine it, we need to know that
Simply, an electrolyte is a substance that can conduct an electric current when melted or dissolved in water. If the physical or chemical process that generates the ions is essentially 100% efficient (all of the dissolved compound yields ions), then the substance is known as a strong electrolyte. If only a relatively small fraction of the dissolved substance undergoes the ion-producing process, it is called a weak electrolyte.
That means, all electrolytes can conduct electricity, and they can be identified as strong or weak by measuring the electrical conductance of an aqueous solution containing the substance.
Finally, another common similarity for electrolytes is that they can conduct electricity.
ANSWER:
Another common feature of all electrolytes, apart from being soluble in water, is that they can all conduct electricity, either to a large or small extent.
The first ionization energy of magnesium is 738 kJ/mol. A good estimate for the second ionization energy of magnesium is:
Answers
The second ionization energy is always greater than the first one. So, the answer is between 1450and 6900 kJ/mol.
Each ionization energy means the energy that it takes to separate the outer electron from the atom. In this case, the best estimation of the second ionization energy is 6900 kJ/mol because it would take more than double the first ionization energy.
Therefore, the answer is d.
What type(a) of interparticle forces exist between molecules in a pure sample of CH2F2? A) induced dipole attractionsB) dipole-dipole forcesC) H-bonding
Answers
This molecule will have dipole-dipole forces.
Answer: B
what type of bonding results from the force of attraction between positively and negatively charged ions?
Answers
ANSWER
Ionic bonding
EXPLANATION
Ionic bonding is also known as electrovalent bond, this is a type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound
The charge are always negative and positive charge.
Therefore, the type of bonding is called ionic bonding
Nitrogen gas and oxygen can combine to produce nitric oxide such a reaction absorbs 88KJ of heat from the surroundings, how many grams of nitrogen gas do you predict were consumed in the reaction?N2 + O2 -> 2NO. AH=180kJ
Answers
N2 (g) + O2 (g) =======> 2 NO (g) AH = 180kJ
Don't forget to balance the reaction.
This is an endothermic reaction because it needs heat to produce and the sign + of AH
Some reactions absorb enthalpy and others free heat.
We can find the mass of N2 using this enthalpy:
N2 (g) + O2 (g) =======> 2 NO (g) AH = 180kJ
x (grams) AH = 88 kJ
First, we calculate the molecular mass of N2:
Molar mass N2 = 28.01 g / mole so,
1 mol N2 = 28.01 g N2
Now:
28.01 g of N2 ----------------------- absorb 180kJ (stoichiometry)
x (we need this)----------------------- 88 kJ (is real)
[tex]x=\text{ }\frac{88kJ\text{ . 28.01 g N2}}{180kJ\text{ }}=13.69\text{ g of N2}[/tex]What type of model that is a smaller or larger copy of an
object and one that shows key features or interactions?
A. mathematical model
B. physical model
C. conceptual model
D. statistical model
Answers
A physical model is a replica that has been built that is intended to represent the original object. A physical model may be the same size as the real object it represents, smaller, or larger. A scale model is one that is either bigger or smaller than the real thing.
A physical model is a streamlined tangible depiction of an object or phenomenon that has to be studied, typically on a smaller scale.
The model can replicate the relevant physical factors (temperature, waves, speed, etc.) and forecast the unique limits of the circumstance. Before starting the last stages of a project, these constraints can be considered, tested, and solutions implemented.
Urban planning, naval construction, aeronautics, and other fields involving geometry, thermodynamics, and fluid mechanics frequently use physical models.
To learn more about types of models, please refer:
https://brainly.com/question/28875248
#SPJ1
The options for this question are vaporizing, condensing, freezing, and melting but I’m not sure where to put them. If anyone could help it would be greatly appreciated
Answers
Physical states are different in compounds if we have a change of temperature or pressure, and in certain temperatures we can see a compound undergo a physical change, going from solid to liquid or gas, and the reverse is also possible, as we will see now:
If liquid is turning into solid, then we have a freezing situation
Liquid to solid will be known as melting
Gas to liquid will be known as condensing
Liquid to gas will be known as vaporizing
“Give the chemical formula(s) for carbonic acid, bicarbonate, carbonate, & calcium carbonate. Write the chemical reaction that produces each of these molecules”I’m a bit confused by this question can someone help me out?
Answers
Answer
The chemical formula(s) are:
Carbonic acid is H₂CO₃
Bicarbonate is HCO₃⁻
Carbonate is CO₃²⁻ and
Calcium carbonate is CaCO₃
The chemical reaction that produces each of these molecules are:
CO₂ + H₂O ⇄ H₂CO₃ ⇄ HCO₃⁻ + H⁺ ⇄ CO₃²⁻ + H⁺
Ca²⁺ + CO₃²⁻ → CaCO₃
Explanation
Chemical name Chemical formula
Carbonic acid H₂CO₃
Bicarbonate HCO₃⁻
Carbonate CO₃²⁻
Calcium carbonate CaCO₃
Carbonic acid, (H₂CO₃) is a compound formed in small amounts when its anhydride, carbon dioxide (CO₂), dissolves in water.
Bicarbonate (HCO₃⁻) forms when a positively charged ion attaches to the negatively charged oxygen atoms of the ion, forming an ionic compound.
H₂CO₃, HCO₃⁻, and CO₃²⁻ can be formed as shown in the chemical equation below.
CO₂ + H₂O ⇄ H₂CO₃ ⇄ HCO₃⁻ + H⁺ ⇄ CO₃²⁻ + H⁺
In the presence of a calcium ion, (Ca²⁺) from hard water, carbonate, (CO₃²⁻) will combine with it to form calcium carbonate.
Ca²⁺ + CO₃²⁻ → CaCO₃
Determine the volume (mL) required to prepare each of the following. 190 mL of a 0.300 M HNO3 solution from a 3.75 M HNO3 solution.Express your answer with the appropriate units.
Answers
To solve this problem we have to use the rule of dilutions:
[tex]V1\cdot C1=V2\cdot C2[/tex]Where V1 is the initial volume, C1 is the initial concentration, V2 is the final volume and C2 is the final concentration.
In this case, we need to find V1. Solve the equation for V1 and then replace C1 for 3.75M, V2 for 190mL and C2 for 0.300M:
[tex]\begin{gathered} V1=\frac{V2\cdot C2}{C1} \\ V1=\frac{190mL\cdot0.300M}{3.75M} \\ V1=15.2mL \end{gathered}[/tex]It means that the volume required is 15.2mL.
Which of these characteristics would most help a city make long-term road
and infrastructure plans?
O A. Age structure
OB. Birthrate
C. Growth rate
OD. Death rate
Answers
The characteristics that would help a city to be able to make long term infrastructural plans is the growth rate. Option C
What is infrastructure?The term infrastructure has to do with the facilities that are put up in a city to aide life in a city. It is the process of development in a city that helps a city to be able to aide the inhabitants to live a comfortable life.
It is very important to see that there is a laid down plan for the development of a city or a place and this is very essential to the development of the place and the better life of the people that live in the city.
Now, the infrastructural plans that are made in a city has to factor in the growth rate of the city and this have to be based on the projected growth rate.
Learn more about infrastructural develpment:https://brainly.com/question/359017
#SPJ1
what is the mass of aluminum used if 0.1 moles of aluminum chloride is produced by reacting aluminium with an acid
Answers
The mass of aluminum used if 0.1 moles of aluminum chloride is produced by reacting aluminum with an acid is 2.69 g.
given that :
number of moles of aluminum chloride = 0.1 moles
The balanced reaction is given as :
2Al + 6HCl -----> 2AlCl₃ + H₂
it is clear from the reaction that:
2 moles of Al produce 2 moles of AlCl₃
the mole ratio is 2 : 2
mean moles of Al = 0.1
molar mass of Al = 26.9 g/ mol
mass of Al = number of moles × molar mass
= 0.1 × 26.9
= 2.69 g
Thus, The mass of aluminum used if 0.1 moles of aluminum chloride is produced by reacting aluminum with an acid is 2.69 g.
To learn more about moles here
https://brainly.com/question/14919968
#SPJ1
How is electroplating done?
A. The anode is replaced by the object to be electroplated.
B. A precipitation reaction is used to plate the cathode with an ion.
OC. The metals in the anode and cathode electrodes trade positions.
OD. Ions of one metal are reduced onto a cathode made of a different
metal.
Answers
The process of electroplating is done as follows:
Ions of one metal are reduced onto a cathode made of a different
metal; option D is correct.What is electroplating?Electroplating is the process by which one metal is used to coat the surface of another metal.
Electroplating is done in order to improve the appearance of one metal and is also done in order to protect against corrosion.
The process of electroplating involves electrolysis.
The metal to be coated over the is used as the anode while the metal to be coated is used as the cathode.
Learn more about electroplating at: https://brainly.com/question/15208303
#SPJ1
Answer question number 13. The question is in the image.
Answers
Answer: One carbon atom can make a maximum of 4 covalent bonds. The best option to answer the question is number 4.
Explanation:
The question requires us to choose, among the options given, which one corresponds to the maximum number of carbon bonds that can be formed by one carbon atom.
To answer this question, we can consider the electronic configuration of a carbon atom. Carbon (C) presents atomic number 6, therefore it contains 6 electrons and its electronic configuration can be written as:
[tex]1s^22s^22p^2[/tex]Note that there are 4 electrons in carbon's valence shell (2s2 and 2p2), thus a carbon atom needs additional 4 electrons to achieve stability.
If the atom needs 4 electrons to achieve stability, it means it can make 4 covalent bonds to "acquire" these electrons.
Therefore, the best option to answer the question is number 4.
Name each of the following Acids and Bases:Mg(OH)2
Answers
ANSWER
Mg(OH)2 is a base
STEP-BY-STEP EXPLANATION:
Firstly, we need to define the word "acid and base"
An acid is a substance that will produce hydrogen ion as the only positive ion when dissolved in water
WHILE
A base is a substance that can neutralize the acid by reacting with hydrogen ions to produce salt and water. A base also contain hydroxyl ion (OH)
From the above definition, you will see that Mg(OH)2 is a base
Laboratory balances that measure to the hundredths (0.01g) are calleda) centigram balanceb) milligram balancec) analytical balance
Answers
Answer:
[tex]A[/tex]Explanation:
Here, we want to select the balance used to measure mass to a hundredth accuracy
The kind of balance used in this case is the centigram balance. The measure the mass of a substance to an accuracy of a hundredth which is 0.01g
11. Balance the following acid/base reaction: __Ca(OH)2 + __H2CO3 --> __CaCO3 + __H2O
Answers
Given the unbalanced acid/base reaction expressed as:
[tex]aC_{}a\mleft(OH\mright)_2+bH_2CO_3\longrightarrow cCaCO_3+dH_2O[/tex]The equation is balanced if the number of moles of element at the reactant is equal to that of the product.
Next is to determine the values of the constants a,b, c, and d by equating the number of moles of the element on both sides.
For the Calcium element:
a = c .................................. 1
For the Oxygen element
2a + 3b = 3c + d ......................... 2
For the Hydrogen element
2a + 2b = 2d
a + b = d .......................... 3
For the carbon element
b = c ....................... 4
Substitute equation 1 and 4 into 3 to have:
c + c = d
2c = d
d = 2c ...................... 5
Substitute equations 1, 4, and 5 into the unbalanced equation to have:
[tex]cC_{}a(OH)_2+cH_2CO_3\longrightarrow cCaCO_3+2cH_2O[/tex]Cancel out the constant "c" from both sides of the equation to have:
[tex]\begin{gathered} \cancel{c}C_{}a(OH)_2+\cancel{c}H_2CO_3\longrightarrow\cancel{c}CaCO_3+2\cancel{c}H_2O \\ C_{}a(OH)_2+H_2CO_3\longrightarrow CaCO_3+2H_2O \end{gathered}[/tex]This gives the balanced chemical equation
I need to see how to do 2 and 3
Answers
Answer:
Explanation:
a) Here, we want to get the limiting reactant
The limiting reactant forms less of the product
From the equation of reaction:
4 moles of aluminum gave 2 moles of product
0.32 mol will give x moles of product:
4 * x = 0.32 * 2
x = 0.16 moles
3 moles of oxygen gave 2 moles of product
0.26 mol will give x moles of product
3 * x = 0.26 * 2
x = 0.173 moles
That means that aluminum is the limiting reagent
b) From the question:
6.38 * 10^-3 mol oxygen gives x mol of product
3 moles of oxygen gives 2 moles of product
To get x:
[tex]x\text{ = }\frac{6.38\times10^{-3}\times2}{3}\text{ = }0.00213\text{ mole}[/tex]For aluminium, we do same process:
[tex]x\text{ = }\frac{9.15\times10^{-3}\times2}{4}\text{ = 0.004575 mole}[/tex]c) From the mass available, we need to get the number of moles that could be produced from each
To get the number of moles, we have to divide the mass by the atomic mass of the element
For Aluminium, the atomic mass is 27 amu
That means the number of moles is:
[tex]\frac{3.17}{27}\text{ = 0.1174 mole}[/tex]Now, from the equation of reaction:
4 moles aluminum gave 2 moles oxygen
0.1174 mole aluminum will give:
[tex]\frac{0.1174\times2}{4}\text{ = 0.0587 mole}[/tex]For oxygen, the atomic mass is 16 amu
For molecular oxygen, we have the molar mass as 32g/mol
The number of moles that will react is thus:
[tex]\frac{2.55}{32}\text{ = 0.0796875 mole}[/tex]From the equation of reaction:
3 moles of oxygen gave 2 moles of product,
0.159375 mole will give:
[tex]\frac{0.0796875\times2}{3}\text{ = 0.053125 mole}[/tex]Since the number of moles from oxygen is lesser, it translates to a lesser amount of product and that makes oxygen the limiting reactant in this case
CH3OH-water interactions: What happens when the CH3OH molecules are surrounded by water molecules? Briefly describe their interaction.
Answers
When the CH₃OH molecules are surrounded by water molecules, there are solvent-solute interactions where the solvent water molecules interact with the OH group of the CH₃OH molecules resulting in the dissolution of the molecules in water.
What is solvation?Solvation refers to the interaction between solvent and solute molecules whereby the solvent molecules surround and interact with the polar groups of the solute molecules resulting in the dissolution of the solute in the solvent.
Solvent-solute interactions are common when polar or ionic substances are dissolved in water. For example, when, methanol, CH₃OH is dissolved in water.
Learn more about solvation at: https://brainly.com/question/12568957
#SPJ1
Match the type of radiation to a correct description of it.BetaAlphaGamma?Decreases the atomic number of the atom.?Increases the atomic number of the atom.?Does not change the atomic number of the
Answers
ANSWER
Alpha particle...........> decreases the atomic number of an atom
Beta particles .............> increases the atomic number of the atom
Gamma particle ..........> does not change the atomic number of the atom
EXPLANATION
The three types of radioactive particles are;
1. Alpha particle
2. Beta particle
3. Gamma particle
Alpha particles has a positive charge with atomic number 2 and mass number 4
Any atom that gives off alpha particle, will have it atomic number drops by 2 and its mass number drops by 4
Hence, alpha particle decreases the atomic number of an atom
Beta particles; The atomic number of a beta particle changes, it can either be positive or negative
When an atom gives off a beta particle, the mass number number of the atom remains unchanged but the atomic number increases
Hence, beta particles increases the atomic number of the atom
Gamma particle is a neutral particle and it does not have any charge on it
When an atom gives off a gamma particle, the mass and atomic numbers of the atom remain unchanged
Hence, gamma particle does not change the atomic number of the atom
For the reaction 2NOBr→2NO2+Br2, the rate law is rate =k[NOBr]^2. If the rate of a reaction is 6.5×10−6molL−1s−1, when the concentration of NOBr is 2×10−3molL−1.What would be the rate constant of the reaction?
Answers
Answer:
k = 1.625 mol⁻¹ L s⁻¹
Explanation:
What is given?
rate = 6.5 x 10⁻⁶ mol L⁻¹s⁻¹,
[NOBr] = 2 x 10⁻³ mol L⁻¹.
Step-by-step solution:
We want to find the rate constant, k of the reaction based on the rate law:
[tex]rate=k\cdot\lbrack NOBr]^2,[/tex]so we just have to solve for 'k' and replace the given values:
[tex]\begin{gathered} k=\frac{rate}{\lbrack NOBr]^2}, \\ \\ k=\frac{6.5\cdot10^{-6\text{ }}mol\text{ L}^{-1}s^{-1}}{(2\cdot10^{-3}\text{ mol L}^{-1})^2}, \\ \\ k=1.625\text{ mol}^{-1}L^s^{-1} \end{gathered}[/tex]The answer would be k = 1.625 mol⁻¹ L s⁻¹
Which of the following statements is FALSE:O Density is a measure of how closely packed the atoms of a substance are.O The Density of a substance can change if its location changes.O The Density of steel is greater than the Density of cotton.O To calculate the Density of an object we must know its mass
Answers
Answer
The Density of a substance can change if its location changes.
Explanation
All the statements are TRUE except
The Density of a substance can change if its location changes.
This is because density is an intensive property, that is regardless of the object's shape, size, or quantity, the density of that substance will always be the same. Even if you cut the object into a million pieces, they would still each have the same density.
Therefore, the statement that is FALSE is
The Density of a substance can change if its location changes.