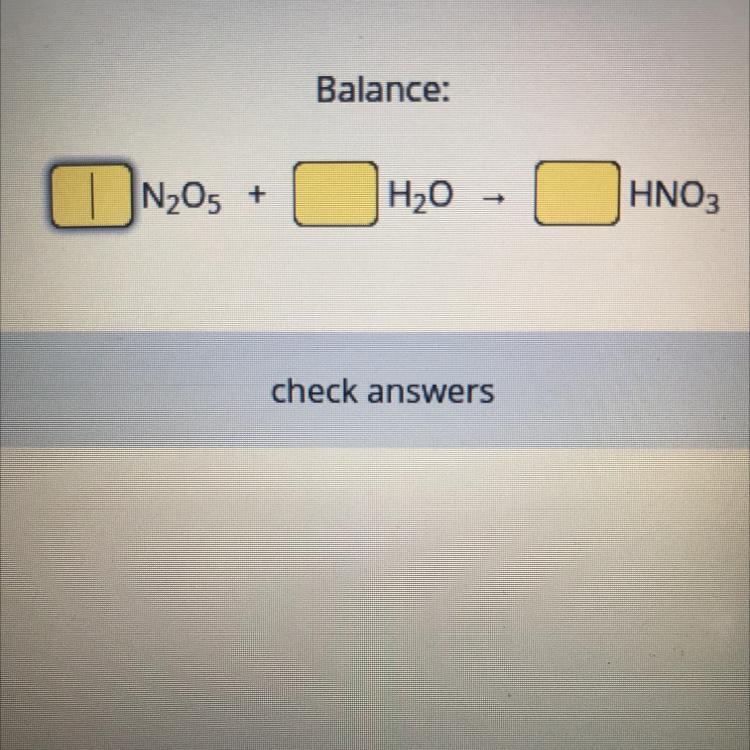
Answers
Answer:
1, 1, 2
Explanation:
1, 1, 2
The coefficients for the N2O5 and H2O are technically 1; if you must provide a value for those reactants, then that would be it.
Related Questions
What is the molar mass of the following compound: C1H3P2
Answers
Stock System naming for NaCl4, SO3, As2F4.
Answers
SO3: SULFITE ION
Why can more sugar be dissolved in hot water than cold water (more solute be dissolved in hot solvent then cold solvent)
Answers
Can Intrusive and Extrusive Igneous rocks form from the same mineral composition?
Answers
EXAMPLE took the test k12
help plsssssssssssssssssssssss
Answers
Answer:
b chemical - thermal and mechanical
Explanation:
energy stored in food in is chemical, so it would only make sense of b) was the answer
1. The multiplicative inverse of 5/9 is.....
a-9/5
b-9/5
c-3/9
d-None of these
Answers
Answer:
None of these cause the correct answer is 9/-5
But The Answer Is -5/9
How many moles are there in 105.69 grams of FeCI2
Answers
Answer:
0.8338395752301793
Explanation:
I used an online converter (grams to moles for FeCl2)
Rubidium (Rb) has two isotopes. Using the data in the table below,
calculate the relative atomic mass of Rb. Give your answer to 3 significant
figures.
Answers
Answer:
85.6
Explanation:
(72 x 85) + (28 x 87)
-----------------------------
100
= 85.56
= 85.6 (3 s.f.)
what happens to the boiling point of hydrocarbon compounds when the number of carbon atom increases
A. Decreases
B. Increases
C. Remains the same
D. None of these
I NEED YOUR ANSWER RN GUYSS PLEASEE
Answers
So therefore your answer choice will increase. (B)
2 HCl (9)
=H2(g) + Cl2 (9)
K = [H][CI]/[HCI)
UK = [H][CI]/2[HCI]
K = 2[HC]/[H]CI.)
K = [HCI)?/[H][1]
Answers
Answer:
(K) = [H2] [Cl2] / [HCl]²
Explanation:
The equation of the reaction is given as;
2 HCl (g) ⇔ H2 (g) + Cl2 (g)
The Equilibrium constant is the ratio of the concentration of the products and reactants raised to the power of their coefficients
Products = H2 and Cl2
Reactants = HCl
Equilibrium constant (K) = [H2] [Cl2] / [HCl]²
What causes the high luster of a metal?
a
excitation of electrons into empty orbitals
b mobility of electrons
C easily ionizable metal atoms
d their low melting points
Answers
Answer:
When light is shone on to the surface of a metal, its electrons absorb small amounts of energy and become excited into one of its many empty orbitals. The electrons immediately fall back down to lower energy levels and emit light. This process is responsible for the high luster of metals.
Explanation:
Your well-wisher :-)
What is the vapor pressure of propanone
at 50°C?
Answers
Answer: If we work it out with direct proportions, the vapor pressure of propanone is 56 degrees Celsius. The atmospheric pressure is about 101.
Explanation: hope this helps.
The vapor pressure of propanone at 50°C is :
- 78° vapor pressure
PropanoneWhen the vapor pressure is specifically work through the extents to a temperature rate it response is intensely subordinate on their possess a mass nature.
The vapor weight of propanone is calculated and shape in degree Celsius in rate.
The rate of vapor when it is calculated by 50°C with propanone its at long last gives us a 78° vapor pressure.
Learn more about "Propanone":
https://brainly.com/question/491528?referrer=searchResults
MORE THEN ONE ANSWER Factors affecting energy transfer
Lesson 2.14-2.15
Question 9 options:
Wind moving over a relatively flat water surface has less area to come in contact with the water, and this reduces energy transfer.
Larger waves have extra spaces and areas to trap air, increasing the transfer of the wind's energy to the water.
Wind moving over a relatively flat water surface has GREATER area to come in contact with the water, and this INCREASES energy transfer.
SMALLER waves have extra spaces and areas to trap air, increasing the transfer of the wind's energy to the water.
Answers
Answer:
1.) Wind moving over a relatively flat water surface has less area to come in contact with the water, and this reduces energy transfer.
2.) Larger waves have extra spaces and areas to trap air, increasing the transfer of the wind's energy to the water.
Explanation:
I got it right on the test.
Wind moving over a relatively flat water surface has less area to come in contact with the water, and this reduces energy transfer and Larger waves have extra spaces and areas to trap air, increasing the transfer of the wind's energy to the water.
What is energy transfer?When energy of any body travels from the one body to the another body, then transfer of energy takes place.
In the oceans, winds transfer their energy to the ocean or to the sea by the interaction with the surface water. When on the surface of ocean or sea up and down movement of water occurs then the interaction of that water with wind takes place and transfer of energy from wind to sea happen. More roughness or more height of the water on the surface of sea observed ten more transfer of energy from wind to sea occur.
Hence, option (1) & (2) is correct i.e. wind moving over a relatively flat water surface reduces energy transfer and Larger waves increasing the transfer of the wind's energy to the water.
To know more about energy transfer, visit the below link:
https://brainly.com/question/2667612
What is the percent composition of Br in CuBr3?
Answers
Answer:
about 79% (79.04369332 to be exact)
Explanation:
Percent composition=(Molar mass of element x amount of it)/Molar mass of compound x 100
Br= 3 x 79.9/303.25 x100=79.04369332
HELP PLEASE!! IF YOU DO AND ANSWER CORRECTLY YOU WILL GET A BRAINLIEST 5 STARSSSS AND A THANKS!! PLEASE PLEASE HELPPP
When scientists communicate about their work, what information should they make sure is included:
1.
2.
3.
4.
5.
6.
7.
Answers
2.) present at conferences
3.) present at universities
4.) inform popular media’s
5.) show data
6.) show hypothesis
7.) and give a theory
Sandban
Grade 8 Science Winter Interim
Question
Q Zoom
Signed in as NOTRE
Q Review Finish Test
Question
>
Normal
ABC
Question 3
Twoiron balls are dropped from a height of 100 meters One Dal Frame A) is not moving when it is dropped and the second ball is moving horizontally at a constant velocity of 10 m/s when it is dropped (Frame B). The balls follow the
trajectories indicated by the arrows and fall for 4,52 s before hitting the ground. (Ignore air resistance)
Frame A
Frame B
1kg
-10%
10kg
Answers
In terms of valence electrons, explain why metals form positive ions.
Answers
Answer:
Explanation:
Metal elements form positively charged ions called cations because they are located on the left side of the periodic table These elements all have valence electrons in an s orbital. These electrons are relatively easy for the atom to lose to achieve a stable octet of electrons in its outermost energy shell.
What kind of mixture is frozen
yogurt with sprinkles, fudge, and
gummy bears? How can you tell
Answers
Answer:
a heterogeneous mixture
Explanation:
I can see all of the different parts of the frozen yogurt, and if I were to pick up a gummy bear, it would come out. Thus, it is not fully/completely mixed with the other components.
It is a heterogenous mixture.
What is the difference between Homogeneous and Heterogeneous Mixtures ?A type of mixtures where all the components are mixed uniformly called as homogeneous mixture.
Here the Particles are distributed in an uniform way, called as solution. Example rainwater, vinegar.
It consist of one phase, It can’t be separated out physically
In heterogenous mixture, all the components are completely and equally mixed, particles can be seen under a microscope.
Here the Particles are distributed non-uniform way, Example: seawater, pizza, etc.
It can be in two or more phases and separated out physically
Learn more about heterogenous mixture, here:
https://brainly.com/question/24898889
#SPJ2
Enzymes in your body act as a catalyst. Thus the role of enzymes is to
O inhibit chemical reactions
o increase the rate of chemical reactions
O help you breathe
decrease the rate of chemical reactions
Answers
Find the mass of 3.27 x 10^23 molecules of H2SO4. Use 3 significant digits
and put the units.
Answers
Answer:
Approximately [tex]53.3\; \rm g[/tex].
Explanation:
Lookup Avogadro's Number: [tex]N_{\rm A} = 6.02\times 10^{23}\; \rm mol^{-1}[/tex] (three significant figures.)
Lookup the relative atomic mass of [tex]\rm H[/tex], [tex]\rm S[/tex], and [tex]\rm O[/tex] on a modern periodic table:
[tex]\rm H[/tex]: [tex]1.008[/tex].[tex]\rm S[/tex]: [tex]32.06[/tex].[tex]\rm O[/tex]: [tex]15.999[/tex].(For example, the relative atomic mass of [tex]\rm H[/tex] is [tex]1.008[/tex] means that the mass of one mole of [tex]\rm H\![/tex] atoms would be approximately [tex]1.008\![/tex] grams on average.)
The question counted the number of [tex]\rm H_2SO_4[/tex] molecules without using any unit. Avogadro's Number [tex]N_{\rm A}[/tex] helps convert the unit of that count to moles.
Each mole of [tex]\rm H_2SO_4[/tex] molecules includes exactly [tex](1\; {\rm mol} \times N_\text{A}) \approx 6.02\times 10^{23}[/tex] of these [tex]\rm H_2SO_4 \![/tex] molecules.
[tex]3.27 \times 10^{23}[/tex] [tex]\rm H_2SO_4[/tex] molecules would correspond to [tex]\displaystyle n = \frac{N}{N_{\rm A}} \approx \frac{3.27 \times 10^{23}}{6.02 \times 10^{23}\; \rm mol^{-1}} \approx 0.541389\; \rm mol[/tex] of such molecules.
(Keep more significant figures than required during intermediary steps.)
The formula mass of [tex]\rm H_2SO_4[/tex] gives the mass of each mole of [tex]\rm H_2SO_4\![/tex] molecules. The value of the formula mass could be calculated using the relative atomic mass of each element:
[tex]\begin{aligned}& M({\rm H_2SO_4}) \\ &= (2 \times 1.008 + 32.06 + 4 \times 15.999)\; \rm g \cdot mol^{-1} \\ &= 98.702\; \rm g \cdot mol^{-1}\end{aligned}[/tex].
Calculate the mass of approximately [tex]0.541389\; \rm mol[/tex] of [tex]\rm H_2SO_4[/tex]:
[tex]\begin{aligned}m &= n \cdot M \\ &\approx 0.541389\; \rm mol \times 98.702\; \rm g \cdot mol^{-1}\\ &\approx 53.3\; \rm g\end{aligned}[/tex].
(Rounded to three significant figures.)
what physical properties does lithium have
Answers
Answer:
Lithium has a melting point of 180.54 C, a boiling point of 1342 C, a specific gravity of 0.534 (20 C), and a valence of 1. It is the lightest of the metals, with a density approximately half that of water. Under ordinary conditions, lithium is the least dense of the solid elements.
Appearance: soft, silvery-white metal
Atomic Number: 3
Atomic Radius (pm): 155
Which statement about Niels Bohr's atomic model is true?
O Higher orbits have lower energies.
Each orbit has a specific energy level.
Electrons can exist in any energy level.
O Orbits close to the nucleus have no energy.
Hurry.
Answers
Answer:
o
Explanation:
Answer:
Each orbit has a specific energy level.
Explanation:
Bohr model
How many grams are in 3.00 moles of carbon
Answers
Answer: Ok I think The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Carbon, or 12.0107 grams. Note that rounding errors may occur, so always check the results. Use this page to learn how to convert between moles Carbon and gram. 12 grams
This means that the atomic mass or atomic weight (12 grams) of carbon is equal to exactly 1 mole of carbon.
hope this helps have a awesome night/day❤️✨
Explanation:
There are 36 grams in 3.00 moles of carbon.
HOW TO CALCULATE MASS:
The mass of a substance can be calculated by multiplying the number of moles by its molar mass. That is;mass (g) = no. of moles (mol) × molar mass (g/mol)According to this question, 3 moles of carbon was given. The mass can be calculated as follows:atomic mass of Carbon = 12g/molMass = 12g/mol × 3molMass = 36g. Therefore, there are 36 grams in 3.00 moles of carbon.Learn more at: https://brainly.com/question/15743584?referrer=searchResults
What is the percent of water in the hydrate whose formula is MgCl2 •6H2O?
Answers
Here is my answer. I hope this is helpful.
If 23.2 g of a given gas occupies a volume of 93.2 L at a particular temperature and pressure, what
mass of the gas occupies a volume of 10.4 L under the same conditions?
Answers
Answer:
2.59g
Explanation:
Using Avagadro's law equation as follows:
V1/n1 = V2/n2
Where;
V1 = initial volume (litres)
V2 = final volume (litres)
n1 = first amount of gas in grams
n2 = second amount of gas in grams
According to the information provided in this question, v1 = 93.2L, v2 = 10.4L, n1 = 23.2 g, n2 = ?
Using V1/n1 = V2/n2
93.2/23.2 = 10.4/n2
Cross multiply
93.2 × n2 = 23.2 × 10.4
93.2n2 = 241.28
n2 = 241.28/93.2
n2 = 2.588
n2 = 2.59g
The mass of the gas is 2.59g that occupies a volume of 10.4 L under the same conditions.
Avogadro's law equation:V₁/n₁ = V₂/n₂
Where
V₁ = initial volume (litres)
V₂ = final volume (litres)
n₁ = first amount of gas in grams
n₂ = second amount of gas in grams
Given:
V₁ = 93.2L,
V₂ = 10.4L,
n₁ = 23.2 g,
To find:
n₂ = ?
On substituting the values:
93.2/23.2 = 10.4/n₂
93.2 * n₂ = 23.2 * 10.4
93.2* n₂ = 241.28
n₂ = 241.28/93.2
n₂ = 2.588
n₂ = 2.59g
Thus, the mass of the gas is 2.59g.
Find more information about Avogadro law here:
brainly.com/question/3491421
Hi! I need some help with this pleaseeee!!
When two objects of different temperatures are placed in contact with one another, eventually: (choose one option from below)
a) both their average kinetic energy and temperature will be the same
b) their average kinetic energy will be the same
c) neither their average kinetic energy and temperature will be the same
d) their temperature will be the same
Answers
Which describes the molecule below?
O A. A lipid with three saturated fatty acids
• B. A lipid with two unsaturated fatty acids and one saturated fatty
acid
• C. A lipid with three unsaturated fatty acids
• D. A lipid with two saturated fatty acids and one unsaturated fatty
acid
Answers
(got the question correct on a test)
A what is matter that is always composed of the same combination of atoms
Answers
Answer:
A substance
Explanation:
Compare the seasons on Titan to the seasons on Earth.
Answers
Plz help, will give brainliest!
Answers
Answer:
C
Explanation:
No change.
The total number moles of products = The Total number of reactants