Answers
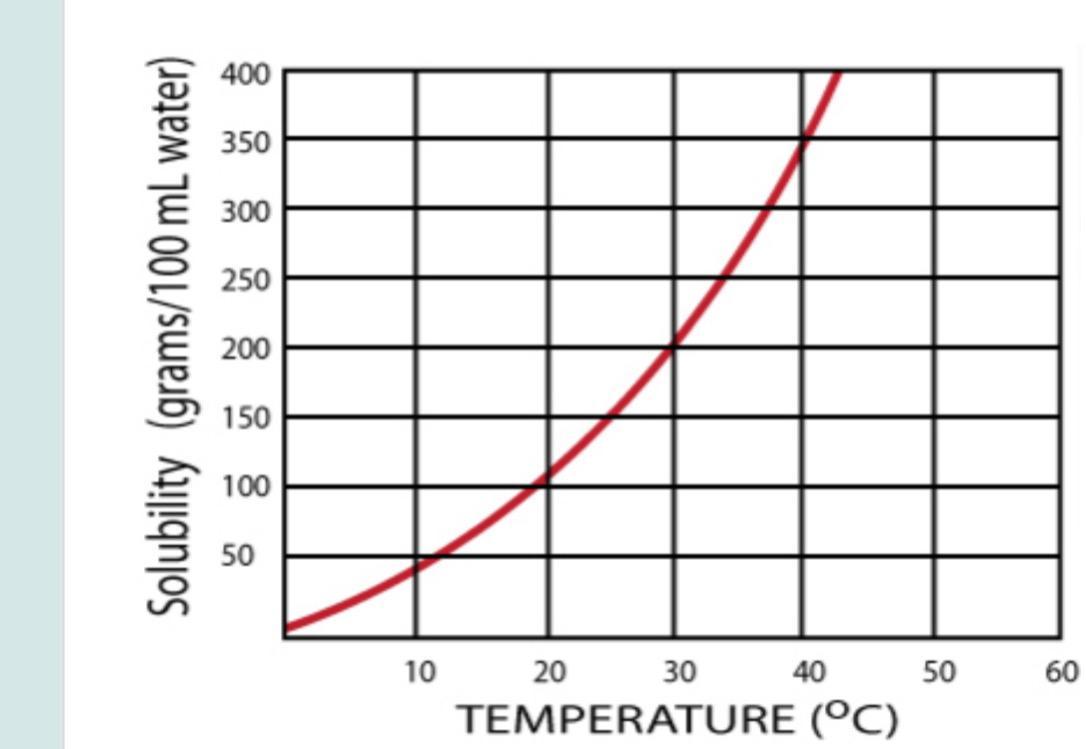
According to the given graph, at 30 degrees, the solution is saturated with 200g of solute per 100mL of water.
It means that if we have 150 grams of solute:
[tex]150g\cdot\frac{100mL}{200g}=75mL[/tex]75mL should be used to have a saturated solution.
Related Questions
Answer the question with the choices Which is not a biotic factor that influences the relationships of organisms in ecosystem?A-Competition among predatorsB- A disease caused by bacteriaC-Invasive speciesD-Weather
Answers
Step 1 - Understanding what is a biotic factor
Biotic, as well as other words with "bio" in them, is related to living organisms. A biotic factot is, therefore, a factor which depends on living organisms. When living organisms shape their environment, as well as other living beings environments, we call it a "biotic factor".
Step 2 - Using the definition to solve the exercise
a) Competition among predators: since this is a consequence of the struggling between two or more living beings, the outcome of it is surely a biotic factor. Individuals can die because they could not find enough food due to competition. A population could thus be modified by this biotic factor, for example.
b) A disease caused by bacteria: a bacteria is a living being and a disease can disastrously kill an entire population, radically changing the ecological relations and the environment. This is another biotic factor.
c) Invasive species: this is also caused by living beings. When a specie invades another species territory, there may be fight among them or just resources sharing. Either way, the ecological relations will be drastically modified. This accounts for a biotic effect as well.
d) weather: this is not solely caused by living beings. In fact, it depends rather poorly on living beings, and much more on Earth's temperature, geographical location an so on. Since the changes caused by weather are not from a living source, this is not a biotic factor.
The answer is thus item d.
Calculate the percent yield if 1.95 g of Ca(OH)2 reacts with excess HCl to produce 2.50 g of CaCl2. Ca(OH)2 + 2HCl → CaCl2 + 2H2OTheoretical yield?Percent yield?
Answers
The first step is to write a balanced chemical equation.
[tex]Ca(OH)_2+\text{ 2HCl}\rightarrow CaCl_2+2H_2O[/tex]The above equation is already balanced.
Now lets write what we have.
Ca(OH)2 = 1.95g
CaCl2 = 2.50g
We already know that HCl is in excess, meaning Ca(OH)2 is the limiting reagent.
%Yield = (actual yield/theoretical yield) x 100
Lets first calculate the theoretcal yield. To find this we need to first calculate the number of moles of CaCl2.
n = m/M where m is the mass and M is the molar mass of CaCl2
n = 2.50g/110,98 g/mol
n = 0.0225 mol
Theoretical Yield is the amount of product that would have been produced if all of the limiting reagent reacted and it was 100% pure. The limiting reagent is used up first in a reaction and controls the amount of product that can be produced.
The actual yield of CaCl2 we were given, which is 2.50g of CaCl2.
For every 1 mole of Ca(OH)2, 1 mole of CaCl2 is produced
n = 1.95g/74.09 g/mol
n = 0.0263 mol
Therefore the number of moles of CaCl2 = 0.0263 mol
mass of CaCl2 = nM
m = 0.0263 mol x 110,98 g/mol
m = 2.92 g
So the theoretical yield of CaCl2 = 2.92g
percentage yield = (2.50g/2.92)*100
percentage yield = 85.62%
A 16%(m/m) solution contains 10 grams of NaOH. It also contains ___ grams of water.
Answers
1) List the known and unknown quantities.
Concentration: 16%(m/m).
Mass of solute: 10 g NaOH.
Mass of solvent: unknown.
2) Set the equation.
Mass percent.
[tex]Mass\text{ }\%=\frac{grams\text{ }of\text{ }solute}{grams\text{ }of\text{ }solvent}*100[/tex]3) Plug in the known values and solve for grams of solvent (water).
[tex]16\%=\frac{10\text{ }g\text{ }NaOH}{grams\text{ }of\text{ }solvent}*100[/tex].
[tex]grams\text{ }of\text{ }solvent=\frac{10\text{ }g\text{ }NaOH}{16}\times100[/tex][tex]grams\text{ }of\text{ }solvent=62.5\text{ }g\text{ }H_2O[/tex]The 16%(m/m) solution contains 62.5 g H2O.
.
In this set of chemical reactions, which is a singlereplacement reaction?Pb(NO3)2(aq) + 2NaCl(aq) → PbCl2(s) + 2NaNO3(aq)CuSO4(aq) + BaCl2(aq) → BaSO4(s) + CuCl2(aq)K2504(aq) + BaCl2(aq) → BaSO4(s) + 2Cl(aq)AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)
Answers
ANSWER
[tex]\begin{gathered} \text{ Zn}_{(s)}+\text{ H}_2SO_{4(aq)}\rightarrow\text{ ZnSO}_{4(aq)}\text{ + H}_{2(g)} \\ \text{ option D} \end{gathered}[/tex]EXPLANATION:
A single replacement reaction is also called a single displacement reaction.
A single replacement reaction is a type of reaction where one element is substituted for another element in a compound during a chemical reaction.
Below reaction is an example of a single replacement reaction
[tex]\text{ A + BC }\rightarrow\text{ B + AC}[/tex]From the given options, you will see that the reaction between Zn and H2SO4 is a single replacement reaction
Zn will displace H2 because it is more reactive than hydrogen
Hence, the correct option is D
A 13.5- L scuba diving tank contains a helium-oxygen (heliox) mixture made up of 24.0 g of He and 5.00 g of O2 at 298 K . Calculate the mole fraction of each component in the mixture.
Answers
13.5- L scuba diving tank contains a helium-oxygen (heliox) mixture made up of 24.0 g of He and 5.00 g of O2 at 298 K then mole fraction of each component in the mixture is ˣHe = 1.15 and ˣO₂ = 7
Mole fraction is the ratio of number of moles one component of a solution or other mixture to the total number of moles representing all of the components
Here given data is
Helium = 24.0 g
Oxygen = 5.00 g
Temprature = 298 K
Here we know the mass of both helium and oxygen, in order to obtain the mole fractions we first need the compute the moles by using their atomic masses 4.00 g/mol and 32.00 g/mol respectively as shown below then
ⁿHe = 24.0 g×1 mol He/4.00g He = 6 mol He
ⁿO₂ = 5.00 g×1 mol of O₂/ 32.00 g = 0.15 mol O₂
Then the mole fraction are
ˣHe = ⁿHe/ⁿHe + ⁿO₂ = 6/6 + 0.15
ˣHe = 1.15
ˣO₂ = ⁿO₂/ⁿO₂ + ⁿHe = 0.15/0.15 + 6
ˣO₂ = 7
Know more about mole fraction
https://brainly.com/question/18721537
#SPJ1
When the temperature of a gas is increased and the pressure is held constant what happens to the density of the gas?
Answers
Answer:
The density of the gas will increase.
Explanation:
From the Charles's law of Ideal gases, we know that, if the pressure is constant, the behavior of the gas will be represented like:
[tex]\frac{V_1}{T_1}=\frac{V_2}{T_2}[/tex]So, if the temperature of a gas is increased, the volume of the gas will decrease, because temperature and volume (in the charles's law, that is with constant pressure) are inversely proportional.
Now, to analyze what happens with the density, we can see the density formula:
[tex]\text{ Density = }\frac{\text{ Mass}}{\text{ Volume}}[/tex]So, if the volume of the gas decrease, the density of the gas will increase, because in the density formula, volume and density are inversely proportional.
Finally, if the temperature of a gas is increased and the pressure is held constant, the density of the gas will increase.
One measure of solubility is concentration in grams per liter. What is the concentration of copper(II) ions, in grams per liter, measured in a 0.200 L sample of water in which 0.00026 g of copper(II) ions are present?
Answers
One measure of solubility is concentration in grams per liter concentration of copper(II) ions, in grams per liter, measured in a 0.200 L sample of water in which 0.00026 g of copper(II) ions are present is 0.0013 g/L means 1.3×10⁻³g/L
Concentration is defined as mass per unit volume
Here given data is
Volume = 0.200 L
Mass = 0.00026 g
We have to calculate concentration = ?
Concentration = mass/volume
Concentration = 0.00026 g/0.200 L
Concentration = 0.0013g/L = 1.3×10⁻³g/L
1.3×10⁻³g/L concentration of copper(II) ions, in grams per liter, measured in a 0.200 L sample of water in which 0.00026 g of copper(II) ions are present
Know more about concentration
https://brainly.com/question/28196125
#SPJ1
10. Which of the following would speed up the rate of a the reaction A(s) + B(aq) → AB(aq) +heata.Add more of reactant Ab. Add more of product ABC. Crush reactant A into a powderd.Cool the reaction vessel
Answers
In this question, we need to determine which option can speed up the rate of a chemical reaction, and there are a few ways possible in order to speed up a reaction, usually we have: Increasing temperature, increasing surface area, increasing the concentration of reactants
In this case, increasing temperature would not make the reaction occur faster, since the reaction is exothermic, therefore there is no need to input energy for the reaction to occur
Increasing the concentration of reactants, is the option A, but there is a small problem, A is a solid compound and B is an aqueous compound, if we add more A, we can speed up the reaction but we can also turn the extra amount of A insoluble, which would not make the reaction to occur faster
The best way, in this case, is to crush reactant A into powder, because this would make the surface area of A to increase, making A and B interact more and then the reaction would occur faster
The best option will be letter C
When the reactants have less enthalpy than the products, then __________.A. the reaction will be completeB. heat energy is absorbedC. heat energy is given outD. None of these
Answers
Chemistry => Thermochemistry => Exothermic and Endothermic Processes
The type of chemical reaction will depend on whether the reaction absorbs or releases energy.
When the reaction releases energy, it is because there is an excess of it and it is called exothermic. This excess occurs because the energy of the products is lower than that of the reactants.
Now, when the products have more energy than the reactants, it means that the reaction needs energy since there is a lack of energy. We call these types of reactions endothermic.
Now, according to the establishment, if the reactants have less energy (or enthalpy), it means that heat must be adsorbed in order to obtain the products that have higher energy (or enthalpy).
Therefore, the answer will be:
B. heat energy is absorbed
What is the value of the equilibrium constant / Kc?
Answers
answer and explanation
in order to determine the equilibrium constant we have to use the ICE method
the initial concentration of PCl5 is given as 0.300M and at this stage, the concentration of PCl3 and Cl2 are zero
the equilibrium constant is 0.5
2 H₂O + electricity → 2 H₂ + O₂15. In which type of cell would this reaction most likely occur?(1) a chemical cell, because it is exothermic(2) an electrolytic cell, because it is exothermic(3) a chemical cell, because it is endothermic(4) an electrolytic cell, because it is endothermic
Answers
Answer:
(4) an electrolytic cell, because it is endothermic.
Explanation:
You can see that we're using electricity to carry out the reaction, so this reaction is occuring in an electrolytic cell.
Remember that electricity is a form of energy. In an exothermic process, the energy is released but in an endothermic process, the energy is being absorb, and the process would required energy.
As you can note, this reaction requires electricity, it required energy, so this would be an endothermic process.
The answer would be (4) an electrolytic cell, because it is endothermic.
number of moles in 16.23 liters of hydrogen sulfide h2s gas at stp?
Answers
The number of moles in 16.23 liters of hydrogen sulfide h2s gas at STP is 0.7245 moles.
Standard temperature and pressure (STP) refer back to the nominal situations within the surroundings at sea stage. these conditions are zero degrees Celsius and 1 ecosystem (atm) of pressure.
A mole is a completely important unit of size that chemists use. A mole of something manner you have got of that aspect, like how having a dozen eggs approach you to have twelve eggs. Chemists should degree the use of moles for very small such things as atoms, molecules, or different debris.
The mole, symbol mol, is the unit of quantity of substance inside the worldwide machine of gadgets. the amount quantity of the substance is a degree of what number of elementary entities of a given substance is in an item or pattern. The mole is defined as containing exactly 6.02214076×10²³ simple entities
calculation:-
mole at STP = volume in liters/22.4
= 16.23 liters /22.4
= 0.7245 moles.
Learn more about moles here:-https://brainly.com/question/15356425
#SPJ9
REACTION; C5H12 + 8O2 5CO2 + 6H2OWhen 2.50 moles of C5H12 react with 12.0 moles of O2, what is the maximum amount of H2O that can be produced in moles?
Answers
The first thing we do is to verify that the equation is balanced. We are going to count the atoms of each element on each side of the reaction.
Reagents side:
Carbon (C) = 5 atoms.
Hydrogen (H) = 12 atoms.
Oxygen (O) = 16 atoms.
Products side:
Carbon (C) = 5 atoms.
Hydrogen (H) = 12 atoms.
Oxygen (O) =16 atoms.
We have the same number of atoms on both sides of the reaction. So, the reaction is balanced. Now, we must determine which is the limiting reagent.
For each mole of C5H12 that reacts, 8 moles of oxygen are needed and 5 moles of CO2 and 6 moles of H2O are produced.
So, if we have 2.5 moles of C5H12 we will need:
Moles of O2 = 2.5 moles of C5H12 x 8 = 20 moles of oxygen.
We only have 12 moles of oxygen, we don't have enough moles to react to 2.5 moles of C5H12. So oxygen is the limiting reagent. So we will calculate based on the number of moles of oxygen.
So, for every 8 moles of oxygen 1 mole of C5H12 reacts, if we have 12 moles of the oxygen we divide this amount by 8 to know how many moles of C5H12 react.
Moles of C5H12 that reacts = 12 moles of oxygen / 8 = 1.5 moles of C5H12
For each mole of C5H12 6 moles of H2O are produced. So the number of moles produced will be:
Moles of H2O produced= 1.5 moles of C5H12 x 6 = 9 moles of H2O
So, the maximum amount of H2O that can be produced is 9 moles.
Now, provide the following information: Molecular formula
Answers
The molecular
Step-by-step explanation:
Molecular formula consists of the chemical symbols for the constituent elements followed by numeric subscripts. The numeric subscripts only tell us the number of atoms of the constituent elements
[tex]\begin{gathered} _{}_{}_{}_{} \\ \text{ Molecular formula of water} \\ H_2O\text{ } \\ \text{The constistuent elements are hydrogen and oxygen} \\ \text{Water has 2 atoms of hydrogen and 1 atom of oxygen} \end{gathered}[/tex]Molecular mass of water can be calculated as follows
H2O
The molar mass of hydrogen is 1
The molar mass of oxygen is 16
Since water has two atoms of hydrogen and 1 atom of oxygen
Molecular mass of water = 2(1) + 1(16)
Molecular mass of water = 2 + 16
Molecular mass of water = 18g/mol
Hence, the molecular mass of water is 18g/mol
If you start with 512 grams of aluminum and 1147 grams of copper chloride to make aluminum chloride and copper, what is the limiting reagent?2Al + 3CuCl -> 2AlCl3 + 3CuA. AlCl3B. CuClC. AlD. Cu
Answers
Chemistry => Stoichiometry => Limiting reactant
The limiting reactant corresponds to the reactant that produces the least amount of moles of products, that is, it will be the reactant that theoretically reacts completely.
To find the limiting reactant we will first calculate the moles of each reactant. For that, we divide the given mass by the respective molar mass of the compounds.
Molar Mass Al = 26.98g/mol
Molar Mass CuCl = 98.999g/mol
Moles Aluminum
[tex]molAl=512g\times\frac{1molAl}{26.98gAl}=19.0molAl[/tex]Moles Copper Chloride
[tex]molCuCl=1147gCuCl\times\frac{1molCuCl}{98.999gCuCl}=11.6molCuCl[/tex]Now, to find the limiting reactant we divide the moles found in each compound between the stoichiometry coefficient from the balanced equation.
So, we will have:
[tex]\begin{gathered} Al\rightarrow\frac{19.0}{2}=9.50 \\ \\ CuCl\rightarrow\frac{11.6}{3}=3.87 \end{gathered}[/tex]The compound with the smallest quotient will be the limiting reactant.
So, the limiting reactant will be CuCl
Answer: B. CuCl
Find the total molarity of the acid? MaVa = MbVb Ma= what you are looking forVa= 100mLMb = 1MVb = 25 mL
Answers
In this question, we have to find the molar concentration of the acid, and the question already provides us with the formula and each required data:
MaVa = MbVb
We have:
Ma = ?
Va = 100 mL
Mb = 1.0 M
Vb = 25 mL
Now we add these values into the formula:
Ma * 100 = 1 * 25
100Ma = 25
Ma = 25/100
Ma = 0.25 M
what’s the mass of NaCL is in 0.625L of 0.207M solution
Answers
ANSWER
The mass of NaCl is 7.5 grams
EXPLANATION
Given that;
The volume of the solution is 0.625L
The molarity of the solution is 0.207 M
Follow the steps below to find the mass of NaCl
Step 1; Find the number of moles using the below formula
[tex]\begin{gathered} \text{ Molarity =}\frac{moles\text{ of solute}}{\text{ volume of solution}} \\ \end{gathered}[/tex][tex]\begin{gathered} \text{ 0.207 = }\frac{\text{ moles of solute}}{\text{ 0.625}} \\ \text{ cross multiply} \\ \text{ moles of solute = 0.207 }\times\text{ 0.625} \\ \text{ moles of solute = 0.1294 mole} \end{gathered}[/tex]Step 2; Find the mass of NaCl using the below formula
[tex]\begin{gathered} \text{ mole = }\frac{\text{ mass}}{\text{ molar mass}} \\ \text{ cross multiply} \\ \text{ mass = mole }\times\text{ molar mass} \end{gathered}[/tex]Recall, that the molar mass of NaCl is 58.44g/mol
[tex]\begin{gathered} \text{ mass = 0.1294 }\times\text{ 58.44} \\ \text{ mass = 7.5 grams} \end{gathered}[/tex]Therefore, the mass of NaCl is 7.5 grams
Name each of the following Acids and BasesH3PO3
Answers
So,
The compound:
[tex]H_3PO_3[/tex]Can be called as:
Phosphorous acid
For each of the given elements, list two other elements with similar chemical properties.
a. iodine (1)
b. barium (Ba)
c. iron (Fe)
Answers
Answer:
Iodine - > Fluorine, Bromine
Barium - > Beryllium, Magnesium
Iron - > Hassium, Osmium
Explanation:
Density is the ratio of a substance's _______ to its volume.Question options:A) weightB) forceC) thicknessD) mass
Answers
Answer
D) mass
Explanation
The density of a substance is a ratio of its mass to volume.
Therefore, density is the ratio of a substance's mass to its volume.
The correct answer is option D) mass
reaction of alkanes with potassium permanganate solution
Answers
Alkanes are paraffins and that means they don't react a lot. SO when potassium permanganate solution is introduced to alkanes, it will not react with KMnO4 & the pink color of the solution shall persist as there is no change in the reaction.
Alkanes do not react with potassium permanganate solution.
Alkanes are the least reactive type of compound. Alkanes are saturated hydrocarbons that are slightly stable due to the presence of only single bonds. Double bonds are absent in them. Two important reactions they undergo are combustion, which is the reaction with oxygen and halogenation, and which is the reaction with halogens. Potassium permanganate is a potent oxidizing agent that dissolves in water to give the purple solution.
Alkanes do not react with potassium permanganate solution because potassium permanganate is an oxidizer and alkanes have no functional groups such as double bonds that can be further oxidized. Thus, alkanes do not react with potassium permanganate.
To learn more about alkanes and potassium permanganate,
https://brainly.com/question/15540819
A balloon is floating around outside your window. The temperature outside is 37 ∘C , and the air pressure is 0.800 atm . Your neighbor, who released the balloon, tells you that he filled it with 3.60 moles of gas. What is the volume of gas inside this balloon?
Answers
Answer:
[tex]114.53\text{ L}[/tex]Explanation:
Here, we want to get the volume of the gas inside the balloon
Mathematically, from the ideal gas equation:
[tex]\begin{gathered} PV\text{ = nRT} \\ V\text{ = }\frac{nRT}{P} \end{gathered}[/tex]where:
P is the pressure which is given as 0.8 atm
V is the volume which we want to calculate
R is the molar gas constant which is 0.0821 L.atm/mol.K
T is the temperature in Celsius which we can convert to K by adding 273 (273 + 37 = 310 K)
n is the number of moles which is 3.6
Substituting the values, we have it that:
[tex]\begin{gathered} \text{ V = }\frac{3.6\text{ }\times0.0821\times310}{0.8} \\ \\ V\text{ = 114.53 L} \end{gathered}[/tex]What are the coefficients when the chemical equation below is balanced?___ Fe + ___ H2SO4 -> ___ Fe2(SO4)3 + ___ H21,1,1,12,3,3,32,3,1,33,2,3,1
Answers
Answer:
2, 3, 1, 3.
Explanation:
Remember that a balanced chemical equation is when we have the same number of elements for reactant and product side.
Let's see the unbalanced equation:
[tex]Fe+H_2SO_4\rightarrow Fe_2(SO_4)_3+H_2.[/tex]You can note that we have:
You can realize that we have Fe, S, and O unbalanced, but if we put '2' moles beside Fe, we will balance Fe, like this:
[tex]2Fe+H_2SO_4\operatorname{\rightarrow}Fe_2(SO_4)_3+H_2[/tex]Now, if we put '3' moles beside H2SO4 we will balance S and O, obtaining 3 moles of S, and 12 moles of O for both sides:
[tex]2Fe+3H_2SO_4\operatorname{\rightarrow}Fe_2(SO_4)_3+H_2[/tex]But H is unbalanced because on the left side we have 6 hydrogens but on the right side we have 2 hydrogens, so if we put '3' moles beside H2, we obtain the balanced chemical equation:
[tex]2Fe+3H_2SO_4\operatorname{\rightarrow}Fe_2(SO_4)_3+3H_2.[/tex]The order of the coefficients is 2, 3, 1, 3.
Answer:
2,3,1,3
Explanation:
I took the test
What is the percent by mass of bromine in the compound MgBr₂?A) 13.2%B) 43.4%C) 56.6%D) 86.8%
Answers
Answer:
D) 86.8%
Explanation:
It necessary to use the molar mass of Br (80g/mol) and MgBr2 (184g/mol). The MgBr2 is the 100%, so with a mathematical rule of three we can calculate the percent by mass of bromine.
In the molecule of MgBr2 there are 2 bromine, so the total mass of bromine it is 160g (80gx2):
[tex]\begin{gathered} 184g-100\% \\ 160g-x=\frac{160g*100\%}{184g} \\ x=86.9\% \end{gathered}[/tex]Finally, the percent by mass of bromine is 86.9% (86.8%).
Which molecules separate during the light reaction of photosynthesis?Carbon dioxide and oxygeno Hydrogen and OxygenООHydrogen and Carbon Dioxide
Answers
Answer
Hydrogen and Oxygen
Explanation
During Light reactions of Photosynthesis, the chlorophyll will be activated by light. This light-activated chlorophyll will split the water molecule to release H+ ions and also oxygen. This process is called Photolysis.
Therefore, the correct option to your question is: Hydrogen and Oxygen
How many grams of hydrogen are in 25 g of (NH4)2SO4?
Answers
ANSWER
The mass of hydrogen in 25g of (NH4)2S04 is 1.5152 grams
EXPLANATION
Given information
[tex]\text{ The mass of \lparen NH}_4)_2SO_4\text{ is 25g}[/tex]To find the grams of hydrogen, please follow the steps below
Step 1: Find the molar mass of (NH4)2SO4
Recall, that
The unit mass of nitrogen is 14u
The unit mass of hydrogen is 1u
The unit mass of sulfur is 32u
The unit mass of oxygen is 16u
[tex]\begin{gathered} \text{ \lparen NH}_4)_2SO_4\text{ = \lbrack\lparen14 + \lparen1}\times4)]\times2\text{ + \lparen32\rparen + \lparen4 }\times16) \\ \text{ \lparen NH}_4)_2SO_4\text{ = \lparen14 + 4\rparen}\times2\text{ + 32 + 64} \\ \text{ \lparen NH}_4)_2SO_4\text{ = 18}\times2\text{ + 32 +64} \\ (NH_4)_2SO_4\text{ = 36 +32 +64} \\ \text{ \lparen NH}_4)_2SO_4\text{ = 132 g/mol} \end{gathered}[/tex]From the calculations, the molar mass of (NH4)2SO4 is 132 g/mol
Step 2: Find the number of moles of the compound
[tex]\begin{gathered} \text{ Mole = }\frac{\text{ mass}}{\text{ molar mass}} \\ \end{gathered}[/tex]Mass = 25g
Molar mass = 132 g/mol
Substitute the given data into the formula above
[tex]\begin{gathered} \text{ Mole = }\frac{25}{132} \\ \text{ Mole = 0.1894 mol} \end{gathered}[/tex]The number of moles of (NH4)2SO4 is 0.1894 mol
Step 3: Find the moles of hydrogen in the compound
There are 8 hydrogen atoms in (NH4)2SO4
Hence, we can find the number of moles of hydrogen below
[tex]\begin{gathered} \text{ Mole of hydrogen = 8 }\times\text{ 0.1894} \\ \text{ Mole of hydrogen = 1.5152 moles} \end{gathered}[/tex]Step 4: Find the mass of hydrogen using the below formula
[tex]\text{ Mole = }\frac{mass}{\text{ molar mass}}[/tex]Recall, that the molar mass of hydrogen is 1.000 g/mol
[tex]\begin{gathered} \text{ 1.5152 = }\frac{\text{ mass}}{\text{ 1}} \\ \text{ cross multiply} \\ \text{ mass = 1.5152}\times\text{ 1} \\ \text{ Mass = 1.5152 grams} \end{gathered}[/tex]Hence, the mass of hydrogen in 25g of (NH4)2S04 is 1.5152 grams
I just want to know if I did this correctly
Answers
First, let's count down the number of carbon. There are six carbon, so the name have "hex"
There are no double or triple bonds, sou hexane.
The compound is a cyclo, so cyclohexane
There are 2 chlorines, one in position 1 and other in position 2 of the cyclo.
The right name is:
1,2-Dichlorocyclohexane
The correctly drawn Lewis structure for CH2CH2 will have ____1. 4 single bonds to carbon2. 2 single bonds to carbon and 1 double bond to a carbon3. 2 single bonds to carbon and 1 singic bond to hydrogen4. 3 single bonds to carbon and 1 single bonds to a carbon5. 2 single bonds to carbon and 2 double bonds to carbon
Answers
Answer:
[tex]2\text{ : 2 single bonds to carbon and 1 double bond to a carbon}[/tex]Explanation:
Here, we want to describe the correct Lewis diagram for the molecule
For the molecule, we will have a double bond between the two central carbon atoms. Then, we will have 2 single bonds from each carbon atom to the hydrogen atoms.
Thus, the correct answer choice here is 2 single bonds to carbon and 1 double bond to a carbon
The correctly drawn Lewis structure for CH₂CH₂ will have 2 single bonds to carbon and one double bond to carbon. Therefore, the correct option is option 2.
A Lewis structure is a representation of a molecule or ion that shows the arrangement of atoms, the bonding between them, and the distribution of valence electrons. It is a simplified way to visualize the electronic structure of a compound.
In a Lewis structure, atoms are represented by their chemical symbols, and valence electrons are represented as dots or lines. Dots are placed around the atomic symbols to indicate non-bonding electrons, and lines represent covalent bonds between atoms. Lewis structures help in understanding the bonding and molecular geometry of a compound, as well as predicting its chemical behavior.
Learn more about Lewis structure, here:
https://brainly.com/question/32988499
#SPJ6
Boron has a greater atomic radius than fluorine true or false?
Answers
Answer:
false trust me gang^^^
Explanation:
6.The ability to do which of the following is a chemical property of a substance?Select one:a. React with oxygen.b. Conduct electricity.c. Be drawn into a wire.d. Be worked into different shapes.
Answers
Answer:
[tex]A\text{ : React with oxygen}[/tex]Explanation:
Here, we want to select the option that represents a chemical property
A chemical property is one in which there is a chemical change
Asides from the first option, the other are physical
By reacting with oxygen, entirely new substances are formed