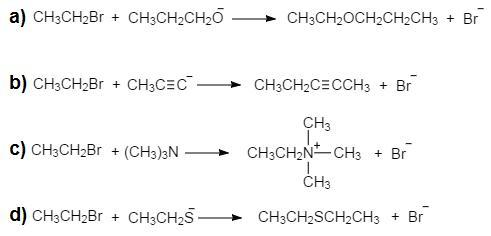
What is the product of the reaction of bromoethane with each of the following nucleophiles? a. CH3CH2CH2O− b. CH3C‚C- c. (CH3)3N d. CH3CH2S-
Answers
The entire bond-forming and bond-breaking process occurs in one step during the SN2 reaction.
When switching from tertiary to secondary to primary alkyl halides, the rate of reaction for SN2 increases. The opposite trend is present for SN1. The pace of reaction for the SN2 goes from primary (fastest) > secondary >> tertiary because steric hindrance rises as we move from primary to secondary to tertiary (slowest).
The alkyl halide and nucleophile both affect the pace of the reaction. It is a second order reaction as a result.
The nucleophile attacks the Br-bearing carbon's reverse side in the subsequent reactions, replacing it.
To know more about nucleophiles, visit:
https://brainly.com/question/28325919
#SPJ4
Related Questions
What is the formula of gas?
Answers
The equations describing these laws are special cases of the ideal gas law, PV = nRT, where P is the pressure of the gas, V is its volume, n is the number of moles of the gas, T is its kelvin temperature, and R is the ideal (universal) gas constant.
By rearranging the ideal gas law, P=n(RTV)=nconst, it can be demonstrated that the pressure of a sample of gas is directly proportional to the number of moles of gas present when volume and temperature are maintained constant. The common measurement of pressure is the pascal (Pa). The kilopascal is the most practical measure for measuring commonplace gas pressures since a pascal is a relatively tiny unit of pressure (kPa). There are 1000 pascals in a kilopascal. The atmosphere is yet another widely used pressure measurement unit (atm).
Learn more about ideal gas law here:
https://brainly.com/question/28257995
#SPJ4
What happens when you mix H2SO4 and nacl?
Answers
When sulfuric acid and sodium chloride are mixed, a reaction occurs that produces sodium bisulphate and hydrogen chloride.
The chemical equation between sulfuric acid and sodium chloride is
2NaCl + H2SO4 → NaHSO4 + 2 HCl(g)
When concentrated Sulphuric acid (H2SO4) is added to Sodium chloride, the formation of Sodium Bisulfite( NaHSO4 ) and Hydrochloric acid (HCL) will take place.The hydrogen chloride gas is released into the air, and can be harmful to breathe.The sodium sulphate is a solid that is left behind in the container.
In this reaction, concentrated Sulphuric acid acts as a strong oxidising agent and Chloride ion acts as a weak reducing agent. With the help of a strong acid, protonation of ions will take place to form Sodium Bisulphate.
To know more about chemical reaction visit here ; https://brainly.com/question/29039149?referrer=searchResults
#SPJ4
Why does calcium chloride melt ice the fastest?
Answers
Because of the low freezing point of calcium chloride it melts the ice fastest.
The calcium chloride provided a better performance at a lower temperature.
As the decrease in temperature, it dissociates into three ions instead of only two ions. These ion allows a way to the bond of water to get rid of the strong bonds.
Also, the hygroscopic properties of calcium chloride makes it attract the moisture from the surroundings.
The faster brine making ability of calcium chloride allows it to melt the ice faster and also when calcium chloride melt the ice it releases heat.
To know mote about calcium chloride, visit,
https://brainly.com/question/8881373
#SPJ4
write the balanced molecular chemical equation for the reaction in aqueous solution for strontium hydroxide and manganese(v) iodide. if no reaction occurs, simply write only nr. be sure to include the proper phases for all species within the reaction.
Answers
Balanced molecular chemical equation for the reaction in aqueous solution for strontium hydroxide and manganese(v) iodide. 5 Sr(OH)₂(aq) + 2 MnI₅(aq) → 2 Mn(OH)₅(s) + 5 SrI₂(aq).
Chemical equations are symbolic representations of chemical processes where the reactants and products are specified in terms of the corresponding chemical formulae. Chemical equations utilize symbols to indicate things like the direction of a reaction and the physical states of the parties involved in the process. The first chemical equation was developed in 1615 by the French chemist Jean Beguin. Chemical equations can be used to depict chemical processes on paper; an example of this is provided below (for the reaction between hydrogen gas and oxygen gas to form water).
2H2 + O2 → 2H2O
The responding entities are written on the left side of the chemical equation, whereas the products produced during chemical reactions are written on the right side, as can be seen in the example presented above.
To know more about chemical equations please refer: https://brainly.com/question/28294176
#SPJ4
Why do atronomer think the univere began with an exploion (the "Big
Bang Theory?"
Answers
Answer:
Explanation: The best-supported theory of our universe's origin centers on an event known as the big bang. This theory was born of the observation that other galaxies are moving away from our own at great speed in all directions as if they had all been propelled by an ancient explosive force.
examine the melting points as you go down a family and across a period. is there a trend? if there is a trend, what is it? a. down a family: b. across a period
Answers
Both the melting and boiling factors lower down the group as the melting points as you go down a family and across a period. is there a trend.
As nicely discovered earlier, the greater delocalised electrons gift and the smaller the radius of the atom, the better the melting factor of the metallic. As we circulate throughout duration three the quantity of delocalised electrons consistent with metallic atom will increase and the radius of the factors decreases. This way the melting factor will increase.
The melting and boiling factors of the Group 1 factors lower on descending the group. This is because of a lower withinside the forces of appeal among the atoms. On crossing a duration withinside the Periodic Table the atomic length decreases. On descending a collection the atomic length will increase.In a duration, the melting and boiling factor first will increase after which decreases.
Read more about melting point:
https://brainly.com/question/40140
#SPJ4
Which group is most likely to react with the halogens?
Answers
why are scientists studying mars
Answers
what is the relationship among electrons, the octet rule, and the oxidation number of a monatomic ion
Answers
The electrons that are shared, lost, or gained are called valence electrons. The charge an ion will have after gaining or losing electrons is indicated by the oxidation number.
According to the octet rule, elements interact with one another to form a noble gas electron configuration by either gaining electrons or losing them. The number of electrons in an element's outermost shell is known as its valency, whereas the number of electrons an element has gained or lost in a compound is known as its oxidation state. The primary distinction between valency and oxidation state is this. Each shell can hold a maximum of half the number of elements in the corresponding period in terms of electrons.
learn more about octet rule here;
https://brainly.com/question/865531
#SPJ4
A plant is left in a protected greenhouse that pumps in fresh carbon dioxide. The plant also has access to sunlight and can make its own sugar. However, the plant is beginning to die. What is most likely missing?.
Answers
For the plant, it can be too hot or too cold. Water is not required by the plant. Carbon dioxide is not necessary for the plant. In order to photosynthesize their food, plants first utilize CO2, but they also use oxygen.
What is photosynthesis?
The plant can produce its own sugar and has access to sunlight. The plant can produce its own sugar and has access to sunlight. But the plant is starting to wither. The process by which plants convert carbon dioxide, water, and sunshine into oxygen and sugar-based energy is known as photosynthesis. Once they have carbon dioxide and water, they can use solar energy to create food. Another gas called oxygen is a byproduct of the production of plant nourishment. The leaves emit this oxygen into the atmosphere.
To learn more about atmosphere from given link
brainly.com/question/26767532
#SPJ4
In an electrolytic cell the electrode at which the electrons enter the solution is called the ______ ; the chemical change that occurs at this electrode is called _______.
Answers
In an electrolytic cell the electrode at which the electrons enter the solution is called the cathode; the chemical change that occurs at this electrode is called reduction.
Please rate as the brainliest
The leaves of the electroscope repelled each other when given a negative charge. Do you think it would be possible to get the leaves of the electroscope to attract each other? Explain.
Answers
No it would not work because an insulator stops electrons from moving from moving to atom to atom so there is no way to get a charge to the metal leaves
What is metal leaf electroscope ?
Gold leaf electroscop has two gold leafs suspended from a metal(usually brass) stem in a vacuumed glass jar and connected to a metal cap. The glass is grounded with the help of a metal foil to make it uncharged. It can be used to:
Detect charge: Body under test is touched with the metal cap. If the leaves diverge, the body is charged and if there is no effect on leaves, then the body is uncharged.
To identify the nature of charge: The electroscope is charged by a known body(say positively charged body) and then the body is removed. Next, the body under test is brought in contact with the metal cap. If the leaves diverge further, the body has same charge(positive) and if the leaves come closer to each other, the body has opposite charge(negative).
Identify a body as conductor or insulator: Take two electroscopes. Charge one of the electroscopes so that its leaves will diverge. Then, connect the two electroscopes by the object under test. If the leaves of other electroscope diverge, the body is a conductor and if there is no effect on the electroscopes, the body is an insulator.
To know more about leaf electroscope, visit
brainly.com/question/15768938
#SPJ1
What patterns do you notice in the periodic table?
Answers
In the periodic table, we notice that elements move up or down a column, they are grouped together into groupings with comparable chemical properties.
The periodic table is reflected in the patterns and trends in its rows and columns. Elements are grouped together into groups that have similar chemical characteristics as they move up or down a column. The atoms trend smaller or larger in size, and their reactivities fluctuate left to right within a row.
Beyond periods and groups, the periodic table divides elements into four groups called blocks: the s block, the p block, the d block, and the f block. These groups provide more information about the arrangement of the electrons, who their likely neighbors are in the table, and which electrons are their valence electrons.
So, Elements are grouped together into groups with similar chemical properties as they progress up or down a column.
To learn more about Patterns of periodic table, here :
https://brainly.com/question/28543363?referrer=searchResults
#SPJ4
which transition state is more stable and why? select an answer and submit. for keyboard navigation, use the up/down arrow keys to select an answer. a the transition state that produces the markovnikov product is more stable because a partial positive charge is forming on tertiary carbon your answer b the transition state that produces the markovnikov product is more stable because a partial positive charge is forming on primary carbon c the transition state that produces the anti-markovnikov product is more stable because a partial positive charge is forming on tertiary carbon d the transition state that produces the anti-markovnikov product is more stable because a partial positive charge is forming on primary carbon
Answers
Answer:
The closer the transition state to the product, the more stable it is. It is because this transition state is closest to the final form of the product, which is usually stable
the compound of which two elements is most likely to involve covalent bonding? electronegativities of unknown elements q 0.9 x 3.0 r 1.0 z 4.0 t 2.8
Answers
The compound of T and X is most likely to involve covalent bonding.
The ability of an atom or functional group to draw electrons to itself is known as electronegativity in chemistry. An atom's electronegativity is influenced by both its atomic number and how far away from its charged nuclei its valence electrons are located.
The differences in the electronegativities are:
Q and Z : 4.0-0.9 = 3.1
R and X : 3.0-1.0 = 2.0
T and Z : 2.8-0.9 = 1.9
T and X : 3.0-2.8 = 0.2
Q and T : 2.8 - 0.9 = 1.9
Smallest difference in electronegativity causes a higher overlap in the orbitals of the shared electrons.
Therefore, the smallest difference between the given elements is between T and X.
To learn more about Electronegativity visit:
https://brainly.com/question/14054766
#SPJ4
if an ideal gas initially at 5 bar and 12.5 l is compressed at constant temperature to 2.5 l, what is the final pressure of the gas? g
Answers
The final pressure of the gas with initial pressure of 5 bar and initial volume of 12.5 L is 25 bar.
The Boyle's law can be used to explain the pressure-volume relationship of an ideal gas at constant temperature. The final pressure can be easily predicted using the relation as shown below if the beginning pressure and volume of an ideal gas are known at a constant temperature with the final volume.
P1V 1 = P2V 2
P 1 denotes the starting pressure, P2 is the ending pressure, V 1 is the starting volume, and V 2 is the ending volume.
The initial pressure P1= 5 bar
The initial volume V1= 12.5 L
The final volume V2= 2.5 L
The final pressure P2=?
P1V1=P2V2
5×12.5= P2× 2.5
P2= 62.5/2.5
P2= 25 bar
For more information on Boyle's law kindly visit to
https://brainly.com/question/21184611
#SPJ4
(1.) A botanist in a laboratory is attempting to grow pothos plants at a faster rate. In order for the plants to grow faster, they must absorb 7.51 x 10° J of energy. What frequency of light is needed to produce a faster growing plant?
(2.) What is the wavelength needed to produce a faster growing plant?
Answers
According to the electromagnetic spectrum and the E= hcυ , the frequency of light is 3.77×10²⁶ s[tex]^-1[/tex] and wavelength of light is 26.5×10[tex]^-[/tex][tex]^28[/tex] m.
What is electromagnetic spectrum?The electromagnetic spectrum consists of electromagnetic radiation which consists of waves made up of electromagnetic field which are capable of propogating through space and carry the radiant electromagnetic energy.
The radiation are composed of electromagnetic waves which are synchronized oscillations of electric and magnetic fields . They are created due to change which is periodic in electric as well as magnetic fields.
In the given problem, frequency of light is calculated as, υ=E/hc which is υ=75.1/6.626×10[tex]^-[/tex]³⁴×3×10⁸=3.77×10²⁶ per second and as wavelength is reciprocal of frequency that is λ=1/υ=1/3.77×10²⁶=26.5×10[tex]^-28[/tex] m.
Thus the frequency and wavelength of light is 3.77×10²⁶ per second and 26.5×10[tex]^-28[/tex] m respectively.
Learn more about electromagnetic spectrum,here:
https://brainly.com/question/23727978
#SPJ1
the half life of a certain radioactive sample is 1 hr. if the mass of the sample is 1 gram at noon, what will be the mass of the sample at 3pm?
Answers
The half life of a certain radioactive sample is 1 hr. if the mass of the sample is 1 gram at noon, what will be the mass of the sample at 3pm?
The half-life is the time it takes for one-half of the radioactive element to decay. The value of the half-life given here is one hour.
After one hour (1 PM) there would only be
(1/2)*(1 g)= 0.5 g of the element remaining.
After three hours (3 PM) there would only be
(1/2)*(1/2)*(1/2)*(1 g) = (1/8)g remaining.
Half-life is the time required for a quantity to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable atoms survive. The term is also used more generally to characterize any type of exponential decay.
To learn more about half life visit:
https://brainly.com/question/24710827
#SPJ4
Give short answers for the following
1. Comment Dalton's atomic theory and the areas in which modifications were
made.
Answers
Dalton's atomic theory was having different postulates. Some of them are:
1. All matters are made up of tiny indivisible particles called atoms.
2. All atoms are identical in mass, size and many other properties.
3. Atoms can be neither created nor destroyed.
4. Atoms can be combined or separated from elements.
But all these theories were modified:
1. Atom can further be divided into 3 parts electron, proton and neutron.
2. Isotopes are members of same atoms family but still have different properties. Isotopes are the number which have same atomic number but different mass number. Example Carbon isotopes.
3. This Theory failed for isobars. Isobars are the elements which have different atomic number but same mass number. Example Ar and Ca
4. This theory failed for Allotropes. Allotropes are the member of same family with different properties. Example Diamond and Graphite which are of carbon family.
To know more about dalton atomic theory follow the following link:
https://brainly.com/question/15507302
Is chlorine polar or nonpolar bond?
Answers
Chlorine is a non-polar bond. An example of a non-polar bond is the bond in chlorine.
Chlorine contains two chlorine atoms. The electrons are shared equally because the electronegativity difference between the two atoms is zero.
When two atoms share electrons evenly, a type of chemical bond known as a non-polar covalent link is created. The number of electrons shared by the neighboring atoms in an atom will therefore be equal. Because of the largest minor difference in electronegativity, the covalent bond is also referred to as nonpolar.
To know more about nonpolar bond, visit;
brainly.com/question/29224615
#SPJ4
how to know which reaction will be reduction and which will be oxidized knowing standard reduction potentials
Answers
The more positive the value is for the standard reduction potential, the more likely the substance is to be reduced.
What is reduction potential?
The reduction eventuality of a species is its tendency to gain electrons and get reduced. It's measured in millivolts or volts. Larger positive values of reduction eventuality are reflective of a lesser tendency to get reduced. We've see before that the complex conformation is accompanied by a drop in the ionic exertion of the essence. Thus, the reduction eventuality of the essence ion decreases upon complexation. The standard reduction eventuality for a free Co 3 ion is 1.853 V, while in perplexed state(Co( NH3) 6) 3, it decreases to 0.1V. Also, a free Fe 3 ion has a standard reduction eventuality of 0.771 V, but ( Fe( CN) 6)−3 has the value of0.36V.To know more about the reduction potential, click the link given below:
https://brainly.com/question/8739272
#SPJ4
Why the reduction potential of Cu2+ is positive?
Answers
When compared to H+/H, a positive result for Cu2+/Cu implies that the reduction occurs more quickly. It is a stronger oxidising agent as a result.
It also implies that Cu is unable to convert acidic H+ ions to H2 using this process. A species' propensity to gain electrons and become reduced is known as its reduction potential. It is expressed in volts or millivolts. Greater reduction potential is indicated by larger positive reduction potential readings. High enthalpy of atomization and low enthalpy of hydration characterise copper. Because the considerable energy required to convert Cu(s) to Cu(aq)2+ is not offset by hydration enthalpy, the value of E(Cu2+/Cu) is unusually positive. Cu2+ has a typical reduction potential of +0.34 For instance, Cu2+ possesses a positive charge and has lost two electrons. oxidise to bring about the loss of electrons in an atom or group of atoms during a chemical process.
To learn more about reduction potential click here https://brainly.com/question/23881200
#SPJ4
a sample of pure fe2o3 contains 14.8 g of oxygen atoms. calculate the total grams of fe2o3. the molar mass of fe2o3 is 159.69 g/mol.
Answers
The total grams of Fe2O3 are 49.2 g Fe2O3.
What is gramme?
The gramme is a unit of mass in the International System of Units (SI) that is equal to one thousandth of a kilogramme. It was originally known as the gramme.
The inorganic substance with the formula Fe2O3 is known as iron(III) oxide or ferric oxide. It is one of the three primary oxides of iron, the other two being the rare iron(II) oxide (FeO) and the naturally occurring iron(II,III) oxide (Fe3O4) found in the mineral magnetite. The primary iron source for the steel industry is Fe2O3, which is the mineral hematite. Acids easily penetrate Fe2O3. Rust is frequently referred to as Iron(III) oxide, and to some extent this term is helpful because of the similarities in composition and a number of its features; nevertheless, in chemistry, rust is a poorly defined substance that is defined as Hydrous ferric oxide.
? g Fe2O3 = 14.8 g O ×1 mol O/15.9994 g O×1 mol Fe2O3/3 mol O×159.69 g Fe2O3/1 mol Fe2O3
= 49.2 g Fe2O3
To learn more about ferric oxide https://brainly.com/question/14611617
#SPJ4
How many electrons are in the outermost shell of the ge4+ ion in its ground state?.
Answers
There are eighteen electrons in the outermost shell of the Ge4+ ion in its ground state.
Electron configuration of germaniumThe electronic configuration shows that the last shell of germanium has four electrons and the d orbital has a total of ten electrons, but the ionic state of the element changes depending on the bond formation.
Electron configuration of the Ge4+ ionThe germanium atom donates two electrons in the 4p orbital and two electrons in the 4s orbital to convert the germanium ion (Ge4+).
Ge – 4e– → Ge4+
The electronic configuration of the germanium ion (Ge4+) is:
1s2 2s2 2p6 3s2 3p6 3d10.
Electrons in the last shell of the Ge4+ ionThe electron configuration shows that the Ge4+ ion has three shells and the last shell has 18 electrons.
In other words, there are eighteen valence electrons in the germanium Ge4+ ion.
Learn more about ions at https://brainly.com/question/14982375
#SPJ4
You are titrating a solution of sodium hydroxide of unknown concentration with a solution of phosphoric acid that has a concentration of 0. 220 m. Starting with 24. 0 ml of the sodium hydroxide solution, you use 19. 4 ml of the acid to titrate the base to completion. Calculate the concentration of the sodium hydroxide solution.
Answers
The concentration of the sodium hydroxide solution after titration is 0.816M.
Calculate the concentration of the base by using the equation for neutralization reaction.
n₁M₁V₁ = n₂M₂V₂
Where,
n₁, M₁ and V₁ are the are the n-factor, molarity and volume of acid (H₃PO₄).
n₂, M₂ and V₂ are the n-factor, molarity and volume of base (NaOH).
Given,
n₁ = 3
M₁ = 0.220 M
V₁ = 24 mL
n₂ = 1
M₂ = ?
V₂ = 19.4 mL
Substituting the values we get,
n₁M₁V₁ = n₂M₂V₂
⇒ 3 × 0.22 × 24 = 1 × M₂ × 19.4
⇒ M₂ = (3 × 0.22 × 24) / 19.4
⇒ M₂ = 0.816 mL
Hence, the value of the concentration of the base is 0.816 mL.
Learn more about concentration of the base from the link given below.
https://brainly.com/question/14924980
#SPJ4
Explain why the spectrum produced by a 1-gram sample of element z would have the same spectral lines at the same wavelengths as the spectrum produced by a 2-gram sample of element z.
Answers
The spectrum produced by a 1-gram sample of element z would have the same spectral lines at the same wavelengths as the spectrum produced by a 2-gram sample of element z because the position of the spectral lines does not depend on the amount of mass. but of the type of atom or molecule that produces them.
What are spectral lines?They are dark or bright lines in a uniform and continuous spectrum, the result of an excess or deficiency of photons in a narrow range of frequencies, compared to other nearby frequencies.
Spectral lines correspond to a certain atomDue to the intrinsic property that each atom has to emit a single spectral line, these lines have been very useful to identify the chemical composition of any medium that allows light to pass through it.
The use of spectroscopyThe spectral lines also depend on the physical conditions of the gas, hence they are used to determine the physical characteristics and chemical composition of stars and other celestial bodies, for which there is no other method of analysis.
Learn more about spectral lines at https://brainly.com/question/27494244
#SPJ4
What are the 3 types of chemical bond which atoms do they form between?
Answers
There are three primary types of chemical bonding such as ionic bond, covalent bond, and metallic bond.
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds. The strength of chemical bonds varies considerably; there are "strong bonds" or "primary bonds" such as covalent, ionic and metallic bonds, and "weak bonds" or "secondary bonds" such as dipole–dipole interactions, the London dispersion force and hydrogen bonding. Strong chemical bonding arises from the sharing or transfer of electrons between the participating atoms.
There are three primary types of bonding: ionic, covalent, and metallic.
An ionic bond is formed when valence electrons are transferred from one atom to the other to complete the outer electron shell.Example of an ionic bond is the formation of sodium fluoride, NaF, from a sodium atom and a fluorine atom.
A covalent bond is formed when the valence electrons from one atom are shared between two or more particular atoms.Covalent bonds usually occur between nonmetals. For example, in water (H2O) each hydrogen (H) and oxygen (O) share a pair of electrons to make a molecule of two hydrogen atoms single bonded to a single oxygen atom.
A metallic bond is formed when the valence electrons are not associated with a particular atom or ion, but exist as a "cloud" of electrons around the ion centres.Metallic bonding is especially common in minerals involving transition metals. Gold, silver, and copper are examples of minerals with metallic bonds.
To know more about Chemical bond visit here ; https://brainly.com/question/12907148?referrer=searchResults
#SPJ4
Omg help I’m being timed
Answers
Write a nuclear equation to describe the neutron-induced fission of Pu-241 to form Kr-89 and Ce-149. Determine how many neutrons are produced in the reaction.
Answers
The number of neutrons produced in the reaction is 5.
Neutrons are the neutral particles present in the nucleus of any atom.
Nuclear equation:
2(1)n₀ + (241)Pu -----> (89)Kr + (149)Ce + 3n₀
In balancing nuclear equations- the sums of the superscripts and the subscripts must be the same on each side of the equation.
On Balancing the superscripts, we get:
2 + 241 = 89 + 149 + x
243 = 238 + x
x = 5
The balanced equation is:
2(1)n₀ + (241)Pu -----> (89)Kr + (149)Ce + 5n₀
Therefore, the number of neutrons produced in the reaction is 5.
To know more about neutrons, refer: https://brainly.com/question/28992636
#SPJ4
What happens to water molecules when they freeze and become solid ice?
Answers
The potential energy of water is decreased when water molecules when they freeze and become solid ice.
Because molecules have energy, they move constantly. Water molecules move more quickly and are essentially in continuous interaction with one another when they are in a liquid state instead of to a solid state.
The potential energy of the liquid decreases as it cools, which causes the molecules to start moving more slowly. The molecules of water bind together at a temperature of about 0°C to produce ice.
Intermolecular hydrogen bonds keep water molecules together, make it a liquid.
Hence, When water molecules freeze and turn into solid ice, their potential energy decreases.
To learn more about Water molecules, Here :
https://brainly.com/question/13436290?referrer=searchResults
#SPJ4