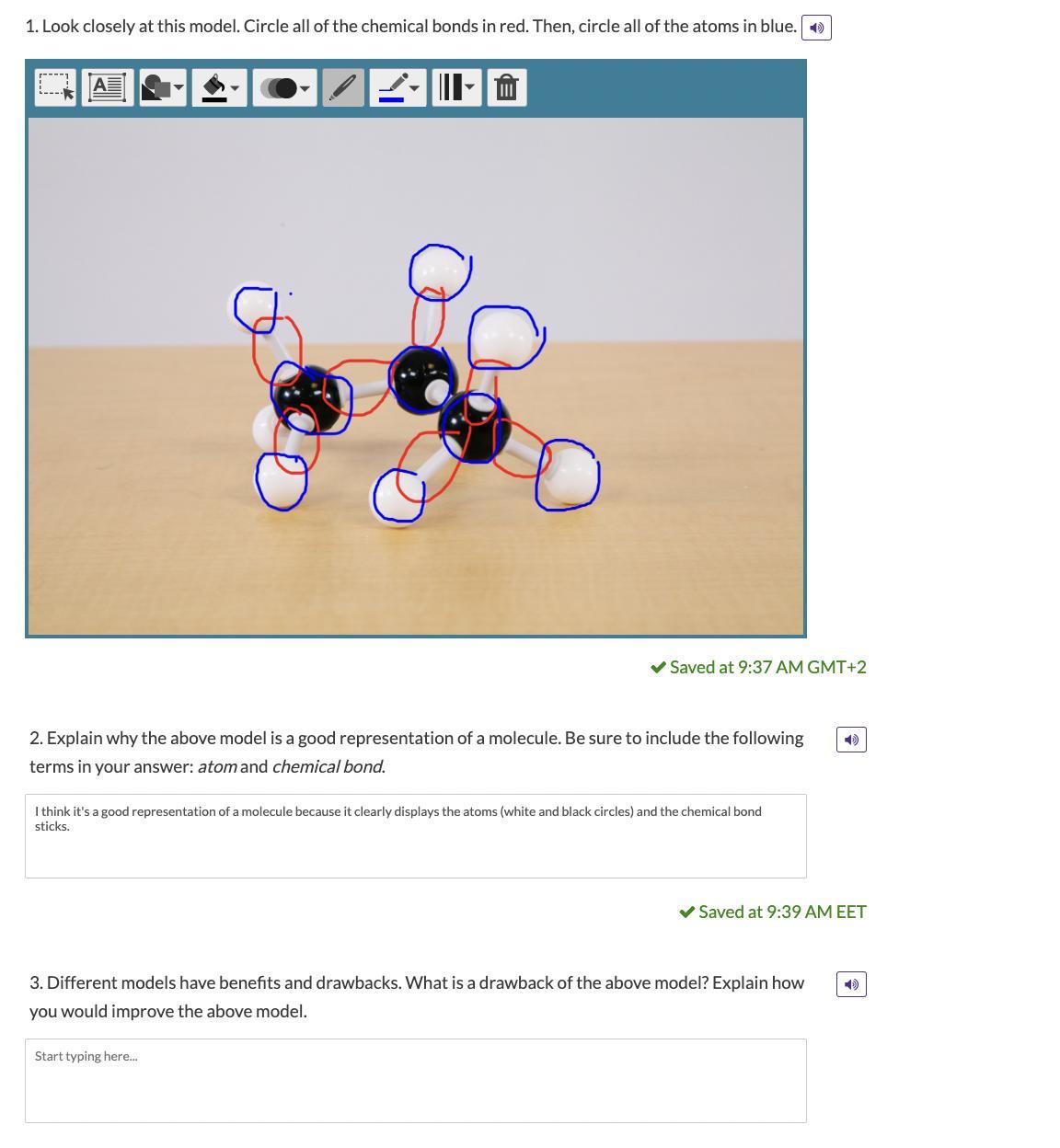
Answers
The above model is a good representation of a molecule because it clearly displays the atoms (white and black circles) and the chemical bond sticks that are present in the atom.
A drawback of the above model is that it does not show clearly the various freedom of movement that may be found in molecules nor does it show the movement of electrons.
The model can be improved by designing it to have relative freedom between the bond to indicate the freedom of movement of bonds.
What are scientific models?Scientific models are structures that are used to represent or explain scientific concepts.
Scientific models may be pictures, three-dimensional objects, illustrations., etc.
Scientists use different models to represent concepts or ideas. For example, the planetary model of the atom, and the ball and stick model of molecules.
The ball and stick model shows the atoms and the bonds that are present in the compound.
Learn more about models at: https://brainly.com/question/18603376
#SPJ1
Related Questions
4) How many moles of a gas occupy 347.8 mL, at 4.369 x 10 atm o and 25.00 C?
Answers
The number of moles of gas occupies 347.8 mL, at 4.369 x 10 atm at 25 degrees C. is 0.0041843391.
What is the mole?The mole is the amount of substance in a system that contains the same number of elementary entities as there are atoms in 0.012 kilograms of carbon 12; it is denoted by the symbol "mol."
The mole, symbol mol, is the SI unit of the material amount of a specified elementary entity, which may be an atom, molecule, ion, electron, any other particle, or a specified group of such particles; its magnitude is set by fixing the numerical value of the Avogadro constant to be exactly 6.022 141 29 1023 when expressed in the SI unit mol-1.
Calculation:We can use the Ideal Gas Law to solve this problem.
PV = nRT
where, Atmosphere pressure P is 4.369 x 10 atm.
Volume V must be expressed in liters, so it will be 0.347.8 L,
and n is the number of moles(n) (and its unknown in this particular question)
R is the universal gas constant (R =.08206 L a t m L/ m o l K ).
T is the temperature in degrees Celsius (degree C + 273 = K, so 25 degrees C + 273 = 298. K).
So we can say,
n = 0.3478*43.49/298*0.08206
n(mole) = 0.0041843391
The number of moles of gas occupies 347.8 mL, at 4.369 x 10 atm at 25 degrees C is 0.0041843391.
To know more about mole, check out;
https://brainly.com/question/921246
#SPJ1
lab: reaction rate student guide
Answers
When changing the concentration of chemical species in an experiment, the rate of chemical reaction can be measured by measuring the amount of the reactants involved or substances produced.
How the measurement of concentration of reactants or products helps in determining the reaction rate.The rate of chemical reactions in a given system is defined as the the speed of rate at which reaction occurs. However, it is also defined as a measure of change in concentration of reactants involved in the chemical reaction or the measure of the change in concentration of substances produced in a reaction per unit time. The time take for this to happen speaks volume of the speed of the reaction rate.
From the context of the task given above, the reaction rate can be measured in a function of the products formed per unit time.
In conclusion, we can now confirm and deduce from step by step explanations above that the concentration of chemical species per unit time is a function of reaction rate.
Complete question:
lab: reaction rate student guide
How would you measure reaction rate in the laboratory when changing concentrations?
Read more on reaction rate:
https://brainly.com/question/12387487
#SPJ1
ΔG°' = -69.5 kJ · mol-1The percent efficiency of oxidative phosphorylation is 35%. Calculate the number of ATP generated by Complex I. Use 1 significant figure.The amount of ATP generated by Complex I is__________________ mol ATP.The equation for the overall reaction that occurs in Complex I is shown as: NADH + H+ + Q ⇌ NAD+ + QH2
Answers
The amount of ATP generated by complex 1 is 14.6 kcal/ mole from the reaction that occurs.
This involves Oxidative phosphorylation. According to this, Most of the free energy released during the oxidation of glucose is retained in the reduced coenzymes NADH and FADH2 , generated during glycolysis and TCA cycle. Electrons are released from NADH and FADH2 and eventually transferred to O2.
NADH + H+ + Q <----> NAD+ + QH2
Gibbs free energy of the reaction is -69.5 KJ/mole , ie that much amount of energy is released because it is the exothermic reaction. We know that oxidative reduction of one NADH produces 91.63 kJ/mole or 21.9 kcal/mole. One NADH upon reduction gives 3 ATP, one ATP is equivalent to 30.54 kJ/mole or 7.3 kcal/mole .
efficiency of the reaction happening in the membrane complex I is 35% only, therefore,
35 = (total energy in output = x)/(total energy input ) * 100
Total energy required or input is = 91.63 kJ/mole + 69.5 kJ/mole (since free energy will be released ) = 161.13 kJ/mole
So,
35 =( x/161.13)*100
x = (35*161.13)/100
x = 56.4 kJ/mole
No of ATP released = 56.40( kJ/mole)/30.54 (kJ/mole) = 2 ATP will be released
Energy released in kcal = 2*7.3 kcal = 14.6 kcal/mole
To learn more about Oxidative phosphorylation please visit:
https://brainly.com/question/8562250
#SPJ4
100 POINTS NEED HELP TO FINISH PLEASE PICTURE BELOW
Answers
Answer:
OMG!!! very long quescions pls ponints give me answer the quescions is "C"
Answer: C
Explanation:
What is the mole ratio of H to N in the ammonia molecule?
Answers
The mole ratio of H to N in the ammonia molecule is 3:1.
What is mole?Mole is defined as the ratio of given mass of substance to the molar mass of that particular substance.
Mathematically,
Mole = Given mass/ Molar mass
As we know that, ammonia have molecular formula NH3.
From this, we can say that one mole of Nitrogen combine with 3 mole of Hydrogen to form a single mole of ammonia.
So, Mole ratio of Nitrogen is given as 1:4.
Mole ratio of Hydrogen is given as 3:4.
Mole ratio of hydrogen to the nitrogen can be given as
Mole ratio of hydrogen/ Mole ratio of Nitrogen
= 3:1
Thus, we concluded that the mole ratio of H to N in the ammonia molecule is 3:1.
learn more about Mole:
https://brainly.com/question/28182370
#SPJ1
what is electron afinity
Answers
Answer: electron affinity is the ability of an atom to accept an electron.
Explanation:
As the name suggests, electron affinity is the ability of an atom to accept an electron. Unlike electronegativity, electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral gas atom. The more negative the electron affinity value, the higher an atom's affinity for electrons.
the columns in the periodic table are called groups what do elements in group 2 have in common
Answers
The valence shells of all the elements in Group 2 have two electrons, giving them an oxidation state of +2.
What is oxidation State ?The potential charge an atom would have if every one of its links to other atoms were fully ionic is known as the oxidation state, also known as the oxidation number. It describes how much an atom in a chemical molecule has been oxidized. The oxidation state can theoretically be positive, negative, or zero.
Six chemical elements from group 2 of the periodic chart make up the alkaline earth metals. They are boron (R), barium (Ba), calcium (Ca), strontium (Sr), magnesium (Mg), beryllium (Be), and radium (Ra).
Thus, The valence shells of all the elements in Group 2 have two electrons, giving them an oxidation state of +2.
To learn more about oxidation state, follow the link;
https://brainly.com/question/11313964
#SPJ1
when a new chiral center is generated from a radical halogenation reaction, the product mixture always consists of one enantiomer in greater quantity than the other enantiomer.
Answers
When a new chirality center is created during a radical halogenation process, an optically active mixture is formed.
What is a mixture?
In chemistry, a mixture is a material composed of two or more different chemicals that are not chemically bonded.A mixture is a physical combination of two or more substances in which identities are preserved and are mixed in the form of solutions, suspensions and colloids.Mixtures are the single product of mechanical mixing or mixing of chemical substances, such as elements and compounds, without chemical bonding or other chemical change, so that each component retains its own chemical properties and composition.Despite the fact that there are no chemical changes to its constituents, the physical properties of the mixture, such as its melting point, may differ from those of the constituents. Some mixtures can be separated into components by physical (mechanical or thermal) means.To know more about mixture, click the link given below:
https://brainly.com/question/21629664
#SPJ4
4) How many moles of a gas occupy 347.8 mL, at 4.369 x 10 atm o and 25.00 C?
Answers
Number of moles, n=0.61.Thus, 0.61 number of moles of a gas molecules are required to occupy 347.8ml×10 atm and 25.00 C
What is ideal gas law?
An ideal gas is an assumed gas composed of many randomly moving point particles that are not affected by interparticle interactions. Thus, ideal gas law is equation of state of hypothetical ideal gas.
Ideal gas law, PV= nRT
Calculation:A ideal gas law equation is
PV=nRT
P is pressure. It's unit should be atmosphere (=4.369×10atm)
V is volume. It's unit should be in litres (=347.8mL= 0.3478 L )
n is number of moles =?
R is universal gas constant (=0.08206 atm. L/mol. K)
T is temperature in K, (= 25 C= 25+273= 298K)
4.369×10 ×0.3478 = n ×0.0826 ×298
Number of mole n = 4.369×10×0.03478/0.0826×25
Thus, the main answer is that the number of moles, n = .61
To know more about idea gas law, click on
https://brainly.com/question/27870704
#SPJ1
A sample of Neon gas occupies 25 mL at 3.2 atm. What would be the volume at
202.6kPa?What law are you using?
O
Charles' Law: V1T2 = V2T1
Gay-Lussac's Law: P1T2 = P2T1
OBoyle's Law: V1P1 = V2P2
Answers
Answer:
Using OBoyle's Law, the volume at 202.6kPa would be 16.25 mL.
Which of these is a product of respiration.
A. CO2
B. O2
C. C2H4
D. H2
Answers
Answer:
A. CO2
Explanation:
Cellular respiration converts oxygen and glucose into water and carbon dioxide. Water and carbon dioxide are by-products.
Answer: A. CO2
Explanation: The body uses oxygen or O2 to help resperate the cells, and CO2 is created as a biproduct and released back into then environment through exhalation.
How many moles of KOH are contained in 50.0 mL of 6.00 M KOH?
Answers
Answer:
The answer is 1.82.
the answer is 0.3 moles
Explanation:
the molarity formula is M=moles/vol in liters
so moles no =M×vol in liters
=6.00×50/1000=0.3 moles
Which statements accurately describe the breaking and forming of bonds during the combustion of methane (CH4)? [Select all that apply.] For each molecule of CH4 consumed, four C-H bonds are broken. For each molecule of CH4 consumed, four O-H bonds are formed. For each molecule of CH4 consumed, six O-O single bonds are broken For each molecule of CH4 consumed, two O-O double bonds are broken. For each molecule of CH4 consumed, two C-O double bonds are formed. For each molecule of CH4 consumed, two C-O single bonds are formed For each molecule of CH4 consumed, two C-H bonds are broken. For each molecule of CH4 consumed, two O-H bonds are formed.
Answers
The correct options are
a). For each molecule of CH4 consumed, four C-H bonds are broken.
b). For each molecule of CH4 consumed, four O-H bonds are formed.
d). For each molecule of CH4 consumed, two O-O double bonds are broken.
e). For each molecule of CH4 consumed, two C-O double bonds are formed.
Define combustion.
Combustion is the name for the chemical reaction in which a material interacts with oxygen to produce heat. Examples include propane, wood, and ethane.
CH4+2O2 → CO2+ 2H2O
4 (C-H), 2 (O=O) are broken and 2 (C=O), 4 (O-H) are formed.
Therefore, the correct options are
a). For each molecule of CH4 consumed, four C-H bonds are broken.
b). For each molecule of CH4 consumed, four O-H bonds are formed.
d). For each molecule of CH4 consumed, two O-O double bonds are broken.
e). For each molecule of CH4 consumed, two C-O double bonds are formed.
To learn more about combustion from the given link.
https://brainly.com/question/13251946
#SPJ4
How many moles are 501 g of OF2?
Answers
Answer:
9.278429604639639
Explanation:
If 123 kJ of heat are evolved when the reaction described below is carried out, the mass of HCl gas (36.46 g/mol) produced is ____ g.
3 Cl2(g) + PH3 (g) → PCl3 (g) + 3 HCl (g) ∆H = - 570.4 kJ
Answers
Based on the heat of reaction, if 123 kJ of heat is evolved when the reaction described below is carried out, the mass of HCl gas (36.46 g/mol) produced is 23.6 g.
What is the heat of a reaction?The heat of a reaction is the amount of heat energy given off or absorbed in the reaction.
During a reaction, energy changes occur as a result of the breaking of bonds in the reactants as well as the formation of bonds in the products.
The sum of the overall energy change that accompanies the formation of products from the reactants gives the heat change of a reaction.
Considering the heat change of the given reaction below:
3 Cl₂ (g) + PH₃ (g) → PCl₃ (g) + 3 HCl (g) ∆H = - 570.4 kJ
The formation of 3 moles of HCl results in the evolution of 570.4 kJ of heat.
If 123 kJ of heat are evolved, then the moles of HCl produced will be:
Moles of HCl produced = 123 kJ * 3 moles / 570.4 kJ
Moles of HCl produced = 0.647 moles
Mass of HCl produced = 0.647 * 36.46
Mass of HCl produced = 23.6 g
Learn more about heat of reaction at: https://brainly.com/question/11631046
#SPJ1
the two molecules below are isomers. a pure sample of which liquid would have the highest boiling point.
Answers
O-dichlorobenzene is the isomer of dichlorobenzene which has highest boiling point.
The boiling point of o-dichlorobenzene is greater than the boiling point of m -and p-dichlorobenzenes due to its greater polar nature. The boiling point order is: o−>m−>p− dichlorobenzene.
What is boiling point?
Boiling point, the temperature at which the pressure exerted by the surroundings on a liquid equals the pressure exerted by the vapor of the liquid; under these conditions, the addition of heat has the effect of converting the liquid into its vapor without raising the temperature.At any temperature, the liquid partially evaporates into the space above it until the pressure exerted by the vapor reaches a characteristic value called the vapor pressure of the liquid at that temperature.As the temperature increases, the vapor pressure increases; at the boiling point, vapor bubbles form in the liquid.To know more about boiling point, click the link given below:
https://brainly.com/question/28986258
#SPJ4
Water molecules sticking to dew
on the grass is an example of
Answers
100 POINTS HELP PLEASEEEEEE WILL GIVE BRAINLIEST
Answers
B. approximate size of the apple
consider the following reaction of nabh4 with a ketone. how many moles of ketone will react with one mole of nabh4?
Answers
The moles of ketone will react with one mole of NaBH₄ are 4 moles
Write the balanced chemical equation for the reaction between the ketone and the NaBH₄ first.
4R₂C = O + NaBH₄→(R₂CH - O)₄BNa
As we can see, in order to react with 4 moles of ketone we need 1 mole of NaBH4.
Acetone, the most popular ketone, is a great solvent for many plastics and synthetic fibers, and it can be employed in a variety of applications.
Acetone is frequently employed as a paint thinner and nail polish remover in domestic settings.
It is employed medicinally in the treatment of acne and for chemical peeling. Nylon is produced using the ketone cyclohexanone.
Sodium borohydride works well as a reducer. It works just as well as lithium aluminum hydride for reducing aldehydes and ketones to alcohols while being less potent. Only sodium borohydride is capable of replacing mercury with H in the second phase of the oxymercuration reaction, but it cannot reduce esters, carboxylic acids, or amides.
Your question is incomplete but this is the general answer.
Learn more about Ketones at https://brainly.com/question/15859478
#SPJ4
When the temperature decreased to
150k, the pressure
K
This shows
us that temperature and pressure
have a.
relationship.
Answers
THER ANSWER IS TRUE OKAY?
using periodic trends, arrange the following atoms in order of increasing electronegativity: se, cl, mg, na se < cl < mg < na se < mg < na < cl na < mg < cl < se mg < na < se < cl na < mg < se < cl
Answers
In order of increasing electronegativity is Cl> Se>Mg> Na.
What is electronegativity ?
An atom's capacity to entice shared electrons to itself is measured by its electronegativity. According to the periodic chart, electronegativity typically rises from left to right throughout a period and falls as you move down a group.
What is atoms ?
An atom is a unit of matter that specifically characterizes a chemical element. One or more negatively charged electrons surround the core nucleus of an atom, which is made up of all of them.
We know in periodic table going from left to right the electronegativity increases and going from up to down the electronegativity decreases.
Now given Se is group 16 3rd period element and Cl is group 17 2rd period element thus Cl is more electronegative than Se ...and Na comes before Mg in the period thus the electronegativity order follows-
Therefore, in order of increasing electronegativity is Cl> Se>Mg> Na.
Learn more about electronegativity from the given link.
https://brainly.com/question/18258838
#SPJ4
The ocean pressure at the depth of the titanic wreck is 40500. kPa. Calculate the ocean pressure in atm. Round your answer to 5 significant digits.
Answers
Answer:
399.80 atm
Explanation:
Since there are 101.3 kPa per every 1 atm, you need to divide the given number by 101.3 to convert the pressure from kPa to atm.
1 atm = 101.3 kPa
40500. kPa 1 atm
--------------------- x -------------------- = 399.80 atm
101.3 kPa
the rtoxic byproducts of amino acid breakdown must be excreted which of th folowing is not of thr mina functions of nitrogen exxcreteion
Answers
Answer:
The toxic by-products of amino acid breakdown must be excreted. Which of the following is NOT one of the main forms of nitrogen excretion
Explanation:
hope this helps
HELP ME WITH MY EARTH/SPACE SCI QUESTIONS! REAL ANSWERS ONLY!
in 2-3 sentences. must answer both questions!
1) How are human activities guided by the availability of fresh water?
2) How can sustainability practices manage freshwater resources?
Answers
1 Human activities may be varied by the addition of freshwater because it can determine/vary what food, crops, and sea animals that come into the area. Additionally, it has determined when civilizations have thrived and begun or unfortunately fail and in extreme measures go extinct.
2 Practices that can help us as human beings include showers, drinking water, washing hands, etc. When we use some of these examples they help us keep clean and live life with a low risk of sickness.
2
Review: Match the gas law to the formula
Dalton's law of Partial Pressure
Combined Gas Law
Gay-Lussac's Law
Charle's Law
Boyle's Law
10 points
au of Partial Pressure states:
PT = P1+ P2+ P3...
P1V1T2 = P2V2T1
P1T2 = P2T1
V1T2 = V2T1
PT = P1+ P2 + P3...
P1V1= P2V2
P1V1= P2V2
Answers
The Dalton' law states the relation P = P1 + P2 + P3..... Boyles' law relates the volume and pressure of a gas by the relation P1 V1 = P2V2.
Charles law - V1 T2 = V2 T1
Gay- Lussac's law - P1 T2 = P2 T2. and
Combined gas law - P1 V1 T2 = P2 V2 T1.
What are gase's laws?There are different laws relating the volume , temperature and pressure of a gas. The earlier among them was Dalton's law of partial pressure. Which states that the total pressure of a gas mixture is the sum of partial pressure of individual gases in it.
P = P1 + P2 + P3 ...
Boyle's law states that at constant temperature, the volume and pressure of a gas is in inverse proportion.
Thus P1 V1 = P2 V2.
Charles's law states that at constant pressures, the volume and temperature of a gas are directly proportional .
Thus, V1 T2 = V2 T1.
Gay-Lussac's law proposed that the pressure and temperature of a gas are in direct proportion.
i.e., P1 T2 = P2 T1.
The Boyle's law, Charles's law and Gay-Lussac's law are combined to form the relation as written below:
P1 V1 T2 = P2 V2 T1.
To find more on gases laws, refer here:
https://brainly.com/question/14717135
#SPJ1
Use the problem below to answer the question:
34 grams of carbon react with an unlimited amount of H2O. The reaction is:
C + H2O → CO + H2
What is the ending substance?
Answers
34 grams of carbon react with an unlimited amount of H₂O. The reaction is C + H₂O → CO + H₂. The ending substance is H₂.
What is carbon ?The chemical element carbon has the atomic number six and the letter C assigned to it. It has a tetravalent atom, which means that four of its electrons can be used to create covalent chemical bonds. It is nonmetallic.
The periodic table's group 14 includes it. The crust of the Earth contains barely 0.025 percent carbon. The nucleus of a carbon atom is made up of six protons and six neutrons, and it is encased in six electrons.
The first two electrons must occupy the innermost atomic orbital according to quantum mechanics, whereas the other four electrons have wave functions that only partially fill the second standard and three second primary orbitals.
Thus, 34 grams of carbon react with an unlimited amount of H₂O. The ending substance is H₂.
To learn more about carbon, follow the link;
https://brainly.com/question/3049557
#SPJ1
Which of the following information is primarily obtained from nuclear magnetic resonance spectroscopy? arrangement of carbon and hydrogen atoms in a compound molecular weight of a compound any conjugated system present in a compound functional groups present in a compound all of these
Answers
The correct option is a). arrangement of carbon and hydrogen atoms in a compound.
Define NMR spectroscopy.
Numerous nuclei have spin, and all nuclei are electrically charged, according to the NMR underlying principle. Energy can go from the base energy to a higher energy level if an external magnetic field is provided.
NMR spectroscopy, also known as nuclear magnetic resonance, has evolved as the method of choice for figuring out the structure of organic compounds. It is the only spectroscopic technique for which a thorough examination and analysis of the entire spectrum is often expected.
Therefore, the correct option is a). arrangement of carbon and hydrogen atoms in a compound.
To learn more about NMR spectroscopy from the given link.
https://brainly.com/question/17564948
#SPJ4
Spell out the full name of the compound.
Answers
Answer:
iupac
Explanation:
hoped this helped:)
What process is being shown in this diagram?
precipitation
metathesis
dissolution
combustion
Answers
May I have help with this question please
Answers
The answer is Sap from a rubber tree
Answer:
c. sap from a rubber tree
Explanation:
most plastics ending in "ene" are synthetic or at least partly synthetic. sap is naturally occurring in nature.