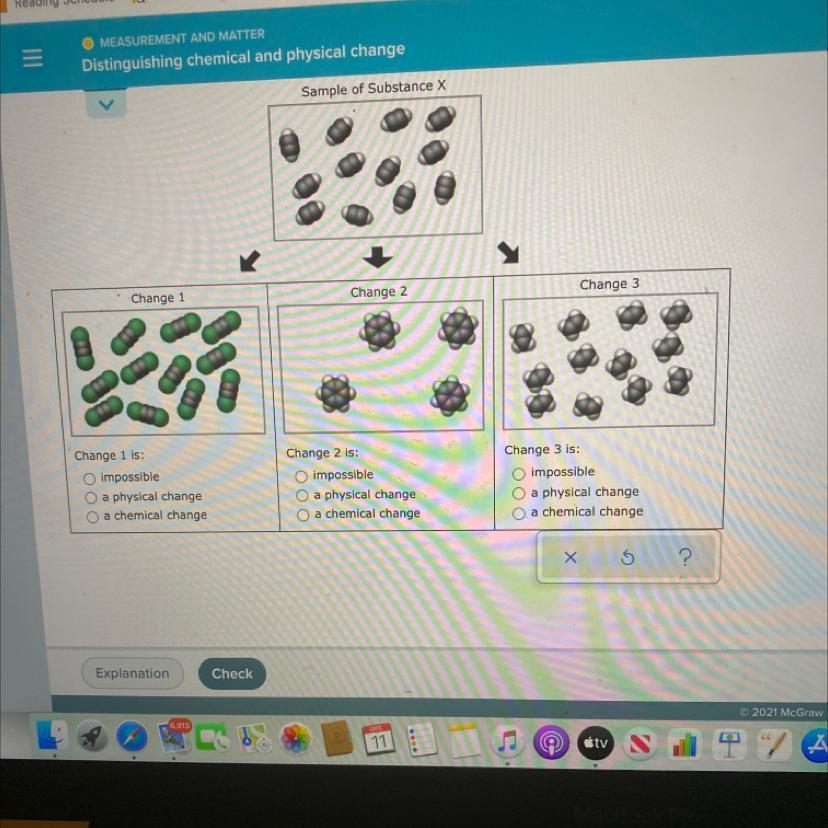
There are four sketches below. The first sketch shows a sample of Substance X. The three sketches underneath it show three different changes to the sample.
You must decide whether each of these changes is possible. If a change is possible, you must also decide whether it is a physical change or a chemical change.
Each sketch is drawn as if the sample were under a microscope so powerful that individual atoms could be seen. Also, you should assume that you can see the
entire sample, and that the sample is in a sealed box, so that no matter can enter or leave.
Sample of Substance X
oli
Change 1
Change 2
Change 3
Change 1 is:
Impossible
a physical change
Change 2 is:
Impossible
a physical change
Change 3 is
Impossible
a physical change
Explanation
Check
Answers
Physical change: a new substance is not produced.
Chemical change: produces a new substance.
The substance x has one white dot, two black dots and one white again.
W - B - B - W.
If we look at the change 1 we can see that we have one green, two black and another green.
Is it possible to have one green atom? What does that mean? It means that we have another atom there. The problem says that the sample is in a sealed box, so that no matter can enter or leave. So in my opinion the first one is impossible.
In the second change I can't decide between physical and chemical. I think it is a chemical change because there is a rearrengement.
In the third change there are 12 molecules, Each molecule has two black atoms and 4 white atoms. In the substance x only had 24 white atoms, but in the change 3 we have 48 white atoms. As we said before the sample is in a sealed box, so that no matter can enter or leave. So it also impossible.
Related Questions
% w/v relates which two units?A.grams and molesB.grams and millilitersC.moles and litersD.temperature and volumeE.milliliters and moles
Answers
Explanation:
The % w/v s the number of grams of solute in 100 mL of solution
So it relates grams of solute with mL of solution.
Answer: B. grams and milliliters
Aluminum bromide can be prepared by reacting small pieces of aluminum foil with liquid bromine at room temperature. The balanced chemical reaction is:2Al(s) + 3Br2(l) → 2AlBr3(s)How many moles of Br2 are needed to produce 5 mol of AlBr3, if sufficient Al is present?
Answers
Based on the mole ratio from the chemical reaction 3 moles of Br2 produces 2 moles of AlBr3. We can set this equation up to determine the unknown:
[tex]\begin{gathered} \frac{2}{3}=\frac{x}{5} \\ 3x=2\times5 \\ x=\frac{10}{3} \\ x=3.3moles \end{gathered}[/tex]3.3 moles of Br2 is needed to reacts with 5 mol of AlBr3.
Atom A and B both have 10 protons A have 10 B has 11 neutrons, which statement is true A,b have the same element, theyvhave differnet elements, if they have the same mass, only atom b is a isotope
Answers
Atom A and B both have 10 protons A have 10 B has 11 neutrons is A and B are isotopes of same element.
Isotopes are the atoms of same elements , that has same number of proton but different number of neutrons , mass no. is depends on neutrons present in an atom. the given situation is :
number of proton number of neutron
Atom A 10 10
Atom B 10 11
Isotopes are the members of the same family of same elements.
Thus, Atom A and B both have 10 protons A have 10 B has 11 neutrons is A and B are isotopes of same element.
To learn more about Isotopes here
https://brainly.com/question/17335691
#SPJ1
How many grams of glucose are required to heat 175 mL of water from 20.0 °C to 25.0°C?
Answers
We must first determine the heat needed to heat 175mL of water. In the working temperature range we can assume that the density of water is 1g/mL, so the mass of water that we must heat will be 175g of water.
Now, the heat we need to heat that mass of water is calculated with the following equation:
[tex]Q=mCp\Delta T[/tex]Where Q is the heat required to heat the water
Cp is the specific heat of water equal to 4186 J/g°C
delta T is the change of temperature = 25°C-20°C=5°C
We replace the know values:
[tex]Q=175g\times4186\frac{J}{g\degree C}\times\frac{1kJ}{1000J}\times5\degree C=3662.75kJ[/tex]Now, to heat the water they tell us that the glucose combustion will take place. We must assume that all the heat generated in the reaction will go to heating the water and that there is no heat loss to the environment.
The combustion reaction of glucose generates 2538.7 kJ/mol. So the moles of glucose needed will be:
[tex]\begin{gathered} molGlucose=givenHeatrequired\times\frac{1molGlucose}{2538.7kJ} \\ molGlucose=3662.75kJ\times\frac{1molGlucose}{2,538.7kJ}=1.44molGlucose \end{gathered}[/tex]We calculate the grams of glucose by multiplying the moles by the molar mass of glucose equal to 180.2g/mol
grams of glucose= 1.44 mol x 180.2 g/mol=260g
Answer: To heat 175 mL of water from 20.0 °C to 25.0°C are required 260g of glucose
could you calculate the percent composition for 15 and the empirical formal for 16
Answers
To solve this question, we need to know the molar mass of Ca. It is 40 g/mol
There are 3 atoms of Ca in Ca3(PO4)2
So the mass of Ca in Ca3(PO4)2 is 3x40 = 120 g
So:
375 grams ---- 100%
120 g --- x
x = 32%
Answer: 32% of calcium.
CaCO3(s) + 2HCl(aq) ---> CaCl2(s) + CO2(g) + H2O(ℓ)What would be the volume of CO2 (at STP) produced from the complete reaction of 10.0 grams of CaCO3?
Answers
The equation is balanced since the number of atoms of each element is the same on each side of the reaction.
Now, we will calculate the number of moles present in 10 grams of CaCO3. We will use the molar mass of CaCO3 for this
[tex]\begin{gathered} lesofCaCO3=gCaCO_3\times\frac{1molCaCO_3}{MolarMass,\text{gCaCO}_3} \\ MolesofCaCO3=10.0gCaCO_3\times\frac{1molCaCO_3}{100.0869gCaCO_3}=0.1molCaCO_3 \end{gathered}[/tex]Assuming that the rest of the reactants are in excess, 0.1 mol of CaCO3 will react and form 0.1 mol of CO2, since the ratio is 1 to 1.
Now to calculate the volume, we can apply the ideal gas law which tells us:
[tex]PV=nR_{}T[/tex]Where,
P is the pressure (STP) =1 atm
V is the volume in Liters
n is the number of moles = 0.1 mol CO2
R is a constant = 0.08206 (atm L)/(mol K)
T is the temperature (STP)= 273.15K
Now, we clear the volume and write the known values:
[tex]\begin{gathered} V=\frac{nR_{}T}{P} \\ V=\frac{0.1\text{mol}\times0.08206\frac{atm.L}{mol.K}\times273.15K}{1atm} \\ V=2.2L \end{gathered}[/tex]The volume of CO2(at STP) produced would be 2.2L
Calculate ∆G° for each reaction using ∆Gf° values: (a) 2Mg(s) + O2(g) → 2MgO(s)
Answers
The free energy of formation of the MgO is −597 kJ/mol.
What is the standard free energy of formation?We know that the free energy of formation is defined as the energy that is evolved or absorbed when a substance is formed from its components under standard conditions. In this case, we have that the magnesium and the oxygen are all pure substances.
It is important that we recall that the free energy of formation of a pure substance is zero. We have the free energy of formation of the magnesium oxide as−597 kJ/mol.
Hence;
Sum of free energy of formation of products - Sum of free energy of formation of reactants
We have;
(−597 kJ/mol) - (0 kJ/mol)
free energy of formation = −597 kJ/mol
Learn more about free energy:https://brainly.com/question/15319033
#SPJ1
Use the equation: 3H2, + N2 —-2NH3, (all are gasses)If you need to make 467L of ammonia gas, how many grams of nitrogen gás do you need to start with?
Answers
1) First, let's rewrite the equation:
3 H2 + N2 ---> 2 NH3
If you need to make 467 L ammonia gas (NH3), let's find out how many moles of it do we need.
For this, we use the molar volume of gases: 22.4L/mol
So:
467 L ------ x mol
22.4 L ----- 1 mol
22.4x = 467
x = 20.8 mol of NH3
2) Now we use the proportion of the chemical equation to find out the quantity in moles of N2 (nitrogen gas):
1 mol of N2 ---- 2 mol of NH3
x mol of N2 ----- 20.8 mol of NH3
2x = 20.8
x = 10.4 mol of N2
3) Now we use the molar mass of N2 to find the quantity in grams that we need to start with:
molar mass N2 = 2x14 = 28 g/mol
Now we use the following equation:
mass = mole × molar mass
mass = 10.4 × 28 = 291.2 grams of Nitrogen Gas
Answer: 291.2 grams of Nitrogen Gas
How do you figure this question out in CHEM 101?What is the mass in grams are in 5.32x10^22 molecules of CO2?
Answers
To answer this question, the first step is to convert the given number of molecules to moles using Avogadro's number:
[tex]5.32\times10^{22}molecules\cdot\frac{1molCO_2}{6.022\times10^{23}molecules}=0.088molCO_2[/tex]Now, use the molecular weight of CO2 to find the mass of this amount of moles:
[tex]0.088molCO_2\cdot\frac{44g}{molCO_2}=3.872g[/tex]The mass of 5.32x10^22 molecules of CO2 is 3.872g.
If compound has molecular weight of 114.22g/mole and its empirical formula is C4H9 FIND its molecular formulaa. C12 H27b) C16 H36c) C8 H18d) C4 H9
Answers
Answer
c) C₈H₁₈
Explanation
Given that:
A compound has a molecular weight of 114.22 g/mol
The empirical formula for the compound is C₄H₉
What to find:
To determine the molecular formula for the compound.
Step-by-step solution:
(Empirica formula)n = (Molecular weight)
(C₄H₉)n = 114.22
(4 x 12.01 + 9 x 1.01)n = 114.22
(48.04 + 9.09)n = 114.22
57.13n = 114.22
Divide both sides by 57.13
n = 114.22/57.13
n = 2
Therefore, the molecular formula for the compound is (C₄H₉)₂ = C₈H₁₈
The correct answer is option c) C₈H₁₈
Finish balancing:__al + 3zn(no3)2 -> __al(no3)3 +__znWhat coefficient does al(no3)3 have in the balanced equation?
Answers
In order to properly balance an equation, we need to make sure that the same amount of elements on the reactants side matches the number of elements on the products side, we can do that by increasing the number in front of each molecule, the so called stoichiometric coefficient. In the reaction from the question we can properly balance by adding the following stoichiometric coefficients
For our question, we have:
2 Al + 3 Zn(NO3)3 -> 2 Al(NO3)3 + 3 Zn
Now this reaction is properly balanced
Al(NO3)3 has the coefficient 2
9. Calculate the molarity of a 400.0 mL solution that contains 41.5 g of NaCl.
Answers
Answer
1.775 M
Explanation
Given:
Volume of solution, V = 400.0 mL
Mass of NaCl = 45.5 g
What to find:
The molarity of the solution.
Step-by-step solution:
Step 1: Convert the volume from mL to L.
This can be done by dividing the volume in mL by 1000.
V = 400.0 mL = (400.0/1000) = 0.4L
Step 2: Convert the mass of NaCl to moles.
The mass can be converted to moles using the mole formula below:
[tex]\begin{gathered} Moles=\frac{Mass}{Molar\text{ }mass} \\ \end{gathered}[/tex]From the periodic table, the molar mass is determined to be = 58.44 g/mol
Putting mass = 41.5 g and molar mass = 58.44 g/mol into the formula, we have:
[tex]Moles=\frac{41.5g}{58.44g\text{/}mol}=0.7101\text{ }mol[/tex]Step 3: Calculate the molarity of the solution using the molarity formula.
Molarity formula is given by:
[tex]Molarity=\frac{Moles}{Volume\text{ }in\text{ }L}[/tex]Moles= 0.7101 mol and V = 0.4 L
Therefore,
[tex]Molarity=\frac{0.7101mol}{0.4L}=1.775\text{ }M[/tex]The molarity of a 400.0 mL solution that contains 41.5 g of NaCl = 1.775 M.
Identify the equation with the elements in BeSO4 in their standard state as the reactants and BeSO4 as the product.
Answers
Answer:
[tex]E[/tex]Explanation:
Here, we want to identify the elements in BeSO4 in their standard state as the reactants and BeSO4 as the product
From what we have, there are 4 atoms of oxygen, 1 atom of Beryllium, and 1 atom of sulfur
In their standard state, oxygen is a gas, Berrylium and Sulfur are solids
The number of elements atom on the reactant side must be equal to that on the product side
Thus, we have the correct choice of answer as E
What is a molecular equation and how do I do that
Answers
option A is is a true molecular equation depicted.
Na2S+2HCl→2NaCl+H2S↑
A molecular equation is a balanced chemical equation in which the constituent ions of the ionic compounds are represented as molecules. Each of a compound's constituent components and the amount of atoms they each possess are listed in the molecular formula. Similar rules apply to the most basic formula: all the constituents are mentioned, but the numbers represent their relative proportions.
There are three different types of equations that may be developed for a reaction involving ionic compounds: molecular equations, full ionic equations, and net ionic equations. In chemistry, each of these equations has a specific function. Because it details exactly which compounds were employed in a process, a molecular equation is useful.
To know more about molecular equation visit : https://brainly.com/question/15329297
#SPJ9
How many grams are in 4.02 x 10^21 atoms of calcium?
Answers
0.268grams
Explanations:According to the Avogadro's constant
1 mole of an atom = 6.02 * 10^23 molecules
Given the following
atoms of calcium = 4.02 x 10^21 atoms
Determine the moles of calcium
[tex]\begin{gathered} moles\text{ of Ca}=\frac{4.02\times10^{21}}{6.02\times10^{23}} \\ moles\text{ of Ca}=0.668\times10^{21-23} \\ moles\text{ }of\text{ Ca}=0.668\times10^{-2} \\ moles\text{ of Ca}=0.00688moles \end{gathered}[/tex]Determine the mass of Calcium
[tex]\begin{gathered} Mass=mole\times molar\text{ mass} \\ Mass=0.00688\times40.078 \\ Mass\text{ of Ca}=0.268gram \end{gathered}[/tex]Hence the required grams in 4.02 x 10^21 atoms of calcium is 0.268grams
I need help on #5-#7
Answers
If 5750 J of energy are added to 455 g of granite at 24 °C. The final temperature of granite is 39.9 °C
Given that :
heat energy = 5750 J
mass of granite = 455 g
initial temperature = 24 °C
specific heat capacity = 0.12 cal / g °C = 0.790 J/ g °C
the expression for the heat capacity is given as :
Q = mcΔT
5750 J = 455 g × 0.790 J/ g °C ( T - 24 °C)
5750 = 359.45 T - 8626.8
T = 39.9 °C
if 5750 J of energy are added to 455 g of granite at 24 °C. The final temperature of granite is 39.9 °C
To learn more about heat capacity here
https://brainly.com/question/28302909
#SPJ1
during the decomposition of 34 gr ammonia into nitrogen and hydrogen 92 KJ are absorbed. How many grams of ammonia are decomposed when 184 KJ are absorbed? write the decomposition reaction
Answers
Explanation:
Ammonia will decompose into nitrogen gas and hydrogen gas according to the following reaction. In that reaction 92 kJ will be absorbed.
2 NH₃ ----> N₂ + 3 H₂ ΔH = 92 kJ
Since the heat is absorbed, the sign is positive and we can add it to the reactants side.
2 NH₃ + 92 kJ ----> N₂ + 3 H₂
When 92 kJ are absorbed 34 g of ammonia are reacted. We can use that relationship to find the mass of ammonia that reacted when 184 kJ where absorbed.
34 g of NH₃ : 92 kJ
mass of NH₃ = 184 kJ * 34 g of NH₃/(92 kJ)
mass of NH₃ = 68 g
Answer: 68 g of ammonia were decomposed.
2 NH₃ ----> N₂ + 3 H₂ ΔH = 92 kJ
Neon (Z=10) has the minimum electron affinity in the 2nd period in the periodic tableIs it true or false? And why?
Answers
ANSWER
False
EXPLANATION
Electron affinity is defined as the energy released by adding an electron to an isolated gaseous atom
Neon has the electronic configuration of 2, 8, This implies that it belongs to period 2 and group 8
Recall, that Neon is a noble gas. Noble gases do not accept or release electrons.
Hence, neon does not have the tendency to accept or release electrons.
Neon as a noble gas has a large positive value for electron affinity
Therefore, the statement is not TRUE
what would be the effect on the melting point of a sample if it were not dried completely after filtering the recrystallized sample?
Answers
It can lower and expand the melting point if the sample is not dried completely.
Impurities may be present in a sample if the drying process is not complete. The melting point is decreased and widened when contaminants are still present in a sample. For instance, if a substance's usual melting point range is 104C to 106C, improper drying could result in the presence of impurities, which would lower and widen the melting point range to something like 85C to 97C.
To know more about melting point click here:
https://brainly.com/question/25777663
#SPJ4
Which of the following is a conclusion that the student can draw based on these observations? The two clear liquids-
Answers
In the process that they describe to us, a mixture initially occurs between these two liquids, when they are mixed, a chemical reaction occurs and one of the products obtained is not soluble in the liquids, therefore a precipitate will form. This type of reaction is called a precipitation reaction.
Therefore, the answer will be:
C) Have gone through a chemical reaction in which a new substance called a precipitate was produced
I’m having trouble figuring out how to do number 13. Iv done some things but still cannot seem to figure it out.
Answers
This is a stoichiometry problem, where we have an initial amount of reactant and we need to find out how much of the product will we end up with, in order to do that we need to:
1. Set up the properly balanced equation, which the question already provided us
2 KClO3 -> 2 KCl + 3 O2
2. Check the molar ratio between the two compounds, which is 2:3, 2 moles of KClO3 will produce 3 moles of O2, but we have 3 moles of KClO3
2 KClO3 = 3 O2
3 KClO3 = x O2
x = 4.5 moles of O2 will be produced from 3 moles of KClO3
3 KClO3
2 KClO3/ 3 O2
4.5 O2
A sample of helium gas initially at 0.90 atm is cooled from 18 degreeC to -48 degreeC at constant volume. What is the final pressure?
Answers
Answer: By using Ideal gas law PV=nRT ,the final pressure is calculated
Explanation:
In thermodynamics a constant volume process is known as isochoric process and in this volume remains constant and pressure varies along with the temperature .
FORMULA USED - PV=nRT
If Volume is constant ,then initial Temperature = 18+273 = 291K
final temperature =225K
initial pressure = 0.90 atm
final pressure = ?
The final pressure comes out to be 0.6958 atm.
To know more about constant volume problems , refer to:https://brainly.in/question/38449186
#SPJ4
Calculate the molar mass of NaHCO3 and round it to 2 decimal places.
Answers
To calculate the molar mass of NaHCO₃, we need to add the molar mass of each of its atoms.
This compound has 1 atom of Na, 1 of H 1 of C and 3 of O.
The molar mass of these atoms can be consulted on a periodic table, and they are:
[tex]\begin{gathered} M_{Na}=22.9898g/mol \\ M_H=1.0079g/mol \\ M_C=12.0107g/mol \\ M_O=15.9994g/mol \end{gathered}[/tex]So, the molar mass of NaHCO₃ is:
[tex]\begin{gathered} M_{NaHCO_3}=1\cdot M_{Na}+1\cdot M_H+1\cdot M_C+3\cdot M_O \\ M_{NaHCO_3}=(1\cdot22.9898+1\cdot1.0079+1\cdot12.0107+3\cdot15.9994)g/mol \\ M_{\mleft\{NaHCO_3\mright\}}=\mleft(22.9898+1.0079+12.0107+47.9982\mright)g/mol \\ M_{\mleft\{NaHCO_3\mright\}}=84.0066g/mol\approx84.01g/mol \end{gathered}[/tex]Determine the [H+] of a solution that has a [OH-] of 6.25*10-12. (Enter answer in expanded format not scientific notation)
Answers
answer and explanation
we are given the [OH] concentration as 6.25*10-12
and we know that
[H+][OH-] = 1.0*10^-14
an so using this relationship we can determine the [H+]
[H+][6.25*10-12] = 1.0*10-14
[H+] = 0.0016M
What is the pressure in a 7.00 L tank with 1.73 grams of hydrogen gas at 375 K?
Answers
Considering the ideal gas law, the pressure in a 7.00 L tank with 1.73 grams of hydrogen gas at 375 K is 26.5987 atm.
Definition of ideal gas lawAn ideal gas is called a hypothetical or theoretical gas, which would be composed of particles that move randomly and without interacting with each other.
An ideal gas is characterized by three state variables: absolute pressure (P), volume (V), and absolute temperature (T). The relationship between them constitutes the ideal gas law, an equation that relates the three variables if the amount of substance, number of moles n, remains constant and where R is the molar gas constant:
P×V = n×R×T
Pressure in this caseIn this case, you know:
P= ?V= 7 Lmass of hydrogen= 1.73 gmolar mass of hydrogen= 1 g/molen= mass of hydrogen× molar mass of hydrogen= 1.73 g÷ 1 g/mole= 1.73 molesR= 0.082 atmL/molKT= 375 KReplacing in the definition of ideal gas law:
P× 2 L = 1.73 moles ×0.082 atmL/molK ×375 K
Solving:
P= (1.73 moles ×0.082 atmL/molK ×375 K)÷ 2 L
P= 26.5987 atm
Finally, the pressure is 26.5987 atm.
Learn more about ideal gas law:
brainly.com/question/4147359
#SPJ1
Which types of electron orbitals will have higher energy than a 4d orbital?A. 4pB. 3sC. 4fD. 5s
Answers
5s. Option D is correct
Explanations:What are electron orbitals?Electron orbitals are area around the nucleus where electron resides. The closer the orbital to the nucleus, the higher the energy level.
Based on the explanation above, 5s will be higher in energy than the 4d orbital.
Perform the following operationand express the answer inscientific notation.5.4x103 x 1.2x107[ ? ]x10[?]Coefficient (green) Exponent (yellow)tCoefficient (green)Enter
Answers
Since the operation is a multiplication, the order in which we made the multiplications doesn't matter, so we can reoprder it:
[tex]5.4\times10^3\times1.2\times10^7=5.4\times1.2\times10^3\times10^7[/tex]Now, we calculate each of them:
[tex]6.48\times10^{3+7}=6.48\times10^{10}[/tex]Why is DNA describes as a blueprint for a cell?
Answers
DNA describes as a blueprint for a cell because it contain the instruction needed for an organism to grow develop and survive
DNA is called a blueprint of life because it contain the instruction needed for an organism to grow develop and survive and reproduce and DNA does this by controlling protein synthesis and protein do must of the work cell and are basic unit of structure and function in the cell of organism
DNA is read by the enzyme RNA polymerase and this enzyme attaches itself to the DNA strand slightly in front of a gene and it then slides along the DNA strand making a copy of the gene only using RNA building blocks instead of DNA building block
Know more about blueprint
https://brainly.com/question/887186
#SPJ1
What physical property can help characterize fragments of glass at a crime scene?
A.
flammability
B.
toxicity
C.
density
D.
volume
Answers
Answer:
I believe it is
C. density
Explanation:
have a nice day!❀
1. A 26.6g sample of mercury is heated to 110.0℃ and then placed in 125g of water in a coffee-cupcalorimeter. The initial temperature of the water is 23.00℃. (c Hg = 0.139 J/g℃). What is the finaltemperature of the water and the mercury? 2. Draw the Lewis bonding structure for PCl3. what would be the hybrid orbitals
Answers
The Lewis structure tells us how atoms are bonded and shows the valence electrons of each element as dots. These valence electrons can be found in the periodic table of the elements, and they coincide with the group number to which the element belongs.
So phosphorus (P) belongs to group 5 and has 5 valence electrons. Chlorine (Cl) is in group 7 and has 7 valence electrons.
The Lewis scheme will be:
The blue dots correspond to the valence electrons of phosphorus, and the purple dots are the electrons of chlorine. The electrons they share can be written with a line.
We have that the geometry of the molecule will be:
Four sp3 type hybrid orbitals are formed, three of which overlap with each of the Cl atoms and the other houses the lone electrons. Sigma type bonds.
Answer: 4 sp3 hybrid orbitals