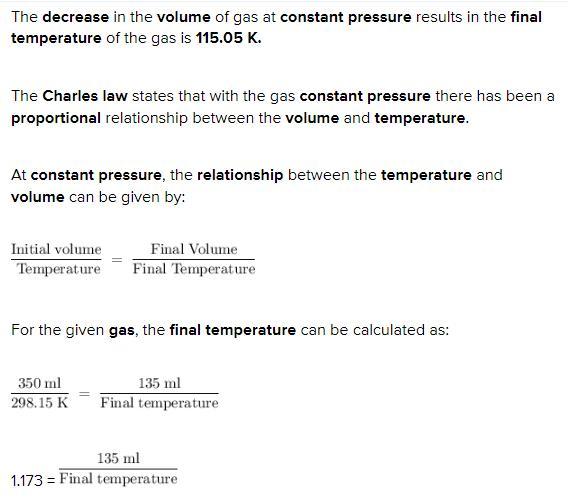
the volume of 350. ml of gas at 25°c is decreased to 135 ml at constant pressure. what is the final temperature of the gas?
Answers
The decrease in the volume of gas at constant pressure results in the final temperature of the gas is 115.05 K.
The Charles law states that with the gas constant pressure there has been a proportional relationship between the volume and temperature.
Gas is a state of matter that has no fixed shape and no fixed volume. Gases have a lower density than other states of matter, such as solids and liquids. There is a great deal of empty space between particles, which have a lot of kinetic energy and aren't particularly attracted to one another.
Kinetic electricity is the energy an object has due to its movement. If we need to boost up an item, then we ought to follow a pressure. applying a force calls for us to do paintings. After work has been achieved, energy has been transferred to the item, and the object might be shifting with a brand new consistent pace.
Learn more about gas here :-
brainly.com/question/24719118
#SPJ4

Related Questions
a 0.85 mol sample of a gas at 27 oc is in a rigid container with a volume of 1.65 l. what is the pressure of the gas in atm?
Answers
The pressure of the gas is 1285.6atm
Since,
T=27°C+273.15=3000.15K
n=0.85mol
V=1.65L
R=8.3144598
From ideal gas law
P= nRT/V
P=0.85×8.3144598×300.15/1.65
P=1285.6 atm
What is the ideal gas law?
The ideal gas law, also known as the general gas equation, is the equation of state for an academic ideal gas.Although the ideal gas law has some limitations, it's an approximation of numerous feasts under numerous conditions. Benoit Paul Émile Clapeyron formulated the ideal gas law in 1834 as a combination of Charles' empirical law, Boyle's law, Avogadro's law, and Gay- Lussac's law. The ideal gas law states that the product of the pressure and the volume of one gram patch of an ideal gas is equal to the product of the absolute temperature of the gas.To know more about the ideal gas law, click the link given below:
https://brainly.com/question/13207402
#SPJ4
Vehicle criteria pollutant (PM, NOx, CO, HCs) emissions: how low should we go?
Answers
Vehicle criterion pollutant emissions: Over the past 30–40 years, vehicle tailpipe emissions of particulate matter (PM), nitrogen oxides (NO x), carbon monoxide, and hydrocarbons (HCs) have increased (CO).
Additionally, hydrocarbons (HCs) have drastically decreased. A chemical or energy that is introduced into the environment and has negative consequences or reduces the usability of a resource is referred to as a pollutant or new entity. These may form naturally (such as minerals or extracted substances like oil) or may be manmade in origin. The term "Carbon Emissions" refers to the total amount of carbon dioxide, expressed in tones, produced when each relevant quantity of fuel is multiplied by the appropriate pollutant emissions factor.
Learn more about hydrocarbons here
https://brainly.com/question/17578846
#SPJ4
What are the 4 types of chemical reactions discuss each with examples?
Answers
There are six types of Chemical Reactions in chemistry, they are:
Combination Reaction: One compound is created by the fusion of two or more compounds.
P + Q → PQ
Decomposition Reaction: In contrast to a combination reaction, a complex molecule disintegrates to produce others that are simpler.
PQ → P + Q
Precipitation Reaction: In contrast to a combination reaction, a complex molecule disintegrates to produce others that are simpler.
P + Soluble salt Q → Precipitate + soluble salt R
Neutralization Reaction: Reactions between acids and bases occur. Typically, salt and water are the end result of this process.
Combustion Reaction: Carbon dioxide and water are created during a combustion event when oxygen and a substance mix. These processes are exothermic, which means that heat is produced.
Displacement Reaction: One ingredient interacts with another element in the compound in a displacement reaction.
To know more about exothermic click on the below link:
https://brainly.com/question/2924714
#SPJ4
Calculate the molar mass of the unknown gas. (remember that the faster the rate of effusion, the shorter the time required for effusion of 1. 0 l ; that is, rate and time are inversely proportional. ).
Answers
The molar mass of the unknown gas is 555.6 g/mol that can be calculated using Grahams' las of effusion.
The molar mass of gas 1= M1
The molar mass of gas 2= M2
As per Graham's law of effusion,
Rate1/Rate2= (M2/M1)1/2
Here, rate 1 is the rate of effusion of the first for the first gas and rate 2 is the rate of effusion for the second gas.
Substituting the given values,
Rate 1= 1.0 L/30 s
Rate 2= 1.0 L/125 s
Then,
[1/30]/[1/125]=(M2/32.0 g/mol)1/2
M2=555.6 g/mol
Therefore, the molar mass of the unknown gas is 555.6 g/mol.
To learn more about molar mass check the link below:
https://brainly.com/question/21334167
#SPJ4
pressure has little effect on the solubility of liquids and solids because they are almost incompressible. True or False
Answers
It is a true statement that; pressure has little effect on the solubility of liquids and solids because they are almost incompressible.
What is compressibility?We know that the term compressibility has to do with the change in the properties of an object by the mere application of pressure on the object that is under study. The factor of compressibility is what is going to determine whether or not a solid, liquid or gas can be able to have a solubility that is affected by the pressure of the substance.
We have to bear in mind that when we are talking about pressure in science that our minds would have to go to the force per unit area of the substance that is under study in each particular case.
We know that the solid and the liquid is not as compressible as the gas because the solid and the gas does have a fixed volume while the gas does not have a fixed volume thus it is going to take on the volume of the container that carries it.
Learn more about compressibility:https://brainly.com/question/23026638
#SPJ1
a 18.6 ml aliquot of 0.201 mh3po4(aq) is to be titrated with 0.269 mnaoh(aq) . what volume ( ml ) of base will it take to reach the equivalence point?
Answers
4.12mL of base will it take to reach the equivalence point.
What is equivalence point?
An equivalence point is a point in a titration when the amount of titrant added is equal to the amount of analyte present in the solution. This is determined by the chemical reaction taking place between the two reactants. It is usually marked by a sudden change in the indicator, such as a color change, which signals that the reaction is complete. The equivalence point is used to determine the concentration of the analyte and can be used to calculate the molarity of the solution. It is important to identify the equivalence point accurately as it is the key to accurate results.
Moles of H3PO4 =18.6/1000 × 0.201M = 0.000374 moles
H3PO4+3NaOH⇒Na3PO4+3H2O
Ratio between H3PO4 and Noah is 1:3
moles Noah needed = 0.00037×3 = 0.00111
V= moles/ molarity =0.00111 / 0.269
V= 0.00412L
V = 4.12 mL
For more information about equivalence point please visit:
https://brainly.com/question/2496608
#SPJ4
which phenomenon that goes unexplained by lewis structures is solved by applying molecular orbital theory?
Answers
The phenomenon of bond order and magnetism in molecules can be explained by applying molecular orbital theory. This theory can help explain why molecules with a higher bond order tend to be more stable, as well as why some molecules possess magnetic properties.
Exploring Bond Order and Magnetism in Molecules Using Molecular Orbital TheoryMolecular orbital theory provides an explanation for phenomena which are not explained by Lewis structures. Bond order and magnetism in molecules can be explained by applying molecular orbital theory. This theory helps to explain why molecules with higher bond orders tend to be more stable and why some molecules possess magnetic properties. The molecular orbital theory states that electrons in a molecule occupy a set of orbitals, which are derived from the atomic orbitals.
Learn more about Lewis structures: https://brainly.com/question/24670696
#SPJ4
Why is the rate of primary succession slow as compared to secondary succession? Your answer should be 3-4 sentences in length.
NEED ANSWERED ASAP
Answers
The rate of primary succession slow as compared to secondary succession because it starts where there is no soil, primary succession moves much more slowly than secondary succession.
Why does primary succession so gradually?Where there has never been an ecosystem, primary succession occurs. Due to the fact that it starts on bare rock, the process is incredibly slow. A retreating glacier doesn't leave any soil behind. A volcanic eruption's lava solidifies into bare rock. Because some cones or seeds are probably still there after the disruption, secondary succession occurs more quickly than primary succession. When native biotic communities have been disrupted, such as when woods have been cut down or burned, when farms have been abandoned, or when lands have been inundated, secondary succession begins. Secondary succession occurs more quickly than primary succession because there is some soil or silt present.
Fertile soil can be created naturally over a period of several hundred to several thousand years. Bacteria and lichens, which can survive without the soil, will likely be the first pioneer species to populate the bare rock.
Learn more about the Primary succession here: https://brainly.com/question/1362887
#SPJ1
convert 0.098 mol RaBr2 to grams
Answers
0.098 x 386 = 37.8 grams RaBr2
Is the following true or false? The subatomic particles that play the key role in determining the properties of an element are electrons.
Answers
Answer: true
Explanation: electrons can help the observer determine things like electronegativity and atomic radius.
how are monomers and polymers related? how are monomers and polymers related? a polymer is chemically identical to a monomer, only larger. a monomer is a small, repeating unit that makes up a polymer. a monomer is made when you condense a polymer through polymerization. a monomer is one strand of a polymer. none of the above
Answers
The relationship between monomers and polymers is that a monomer is a small repeating unit that makes up a polymer.
What is a monomer?A monomer is a molecule of small molecular mass that is linked to other monomers, sometimes hundreds or thousands, through chemical bonds, usually covalent, forming macromolecules called polymers.
What is a polymer?A polymer is a substance composed of large molecules, or macromolecules formed by the union by covalent bonds of one or more simple units called monomers.
Learn more about monomers at https://brainly.com/question/14695306
#SPJ4
What are the four transition effects?
Answers
The four electronic transition effects is :
σ - σˣ transitionn - σˣ transitionπ - πˣ transition n - πˣ transition1) σ - σˣ transition occurs in the saturated hydrocarbon in which only sigma bonds are formed and no non bonding electrons.
2) n - σˣ transition occurs in which non bonding electrons are present.
3) π - πˣ transition occurs in which the unsaturated hydrocarbons contain the double or triple bond.
4) n - πˣ transition , this type of transition occurs in which the non bonding electrons on the hetero atom. the electron excited to the antibonding orbital that is πˣ .
To learn more about transition here
https://brainly.com/question/18156550
#SPJ4
the compound ammonia, nh3, is a weak base when dissolved in water. write the kb expression for the weak base equilibrium that occurs in an aqueous solution of ammonia:
Answers
When dissolved in water, the molecule ammonia, nh3, acts as a weak base. The weak base equilibrium that exists in an aqueous solution of ammonia has a kb expression of 1.8 10 5.
The reaction of the base ammonia joining with water to create ammonium, the conjugate acid, and a hydroxide anion has an equilibrium constant known as Kb (OH-).
The process is as follows: NH3 + H2O --> NH4+ + OH-
Kb= (product of products)/(product of reactants) with units of concentration in molarity or pressure is the formula for any equilibrium constant (for gasses only). Water and other pure chemicals are not part of the recipe.
Kb equals (product of products)/ (product of reactants)
1.8×10^−5 = (OH-)(NH4+) / (NH3) (NH3)
To learn more about equilibrium click here https://brainly.com/question/29224051
#SPJ4
Calculate the mass of 2×10^25 molecules of water
Answers
Answer:6.023*10²³ / 2*10²⁵
To find the mass of a one mole of a specific molecule, add the atomic masses of each of its component atoms.\
How do you determine the bond between two elements?
Answers
Step 1: Look at the atoms in the molecule and look up their electronegativity. Use the following table attached below to look up the electronegativities of the first 20 elements.
Step 2: Calculate the difference between the electronegativities of the atoms across each bond by subtracting the lower electronegativity from the higher electronegativity.
Step 3: Determine the bond type based on the differences in electronegativities. Ionic bonds have differences in electronegativity of 1.7 and higher. Polar-covalent bonds have differences in electronegativity of between 1.7 and 0.4. Covalent bonds have differences in electronegativity of 0.4 or lower.
To know more about electronegativity visit here ; https://brainly.com/question/17762711?referrer=searchResults
#SPJ4
What are 3 things a supplier label must include?
Answers
The 3 things a supplier label must include is the product identifier , supplier identifier and the hazard statement.
The supplier label must include the product identifier that is the name of the product . it must include the supplier identifier that is the name of company that sold the product. The supplier label must includes the hazard statements that is the precaution we have to take while handling the product. the hazard pictogram should be included in the supplier label. the precautionary statement is helpful to handle the product.
Thus, the product identifier , supplier identifier and the hazard statement should be included in the supplier label.
To learn more about label here
https://brainly.com/question/29545525
#SPJ4
2. (4 pts.) use the gibbs phase rule to find the degree of freedom in the following systems: (a) compressed liquid water; (b) pure co2 at its triple point; (c) a liquid phase and a gas phase, each of which contains a homogenous mixture of ammonia and water; (d) a homogenous and gaseous mixture of n2 and o2;
Answers
P+n=c+1 is the Gibbs phase rule. n is the number of thermodynamic degrees of freedom (F) in the system.
It describes the relationship between the number of phases p and components c in a specific alloy under equilibrium circumstances at constant pressure.
P = 1 for pure water in a single phase. S is 1 and r is 0 if we treat the water as a single species, H2O. F of liquid water equals 2+1+0-1 = 3 (F=2+s-r-P).
For CO2, s=4, r=2, and P=2, F =2+4-2-2 = 2 is the result.
For a gas phase and a liquid phase that are both uniformly mixed with ammonia and water F = 6
S=0 r=1 P=1 F = 2 for a uniform and gaseous combination of n2 and o2.
To learn more about degree of freedom click here https://brainly.com/question/16254305
#SPJ4
how many molecules are in 9.4 moles of ibuprofen?
Answers
The number of molecules in 9.4 moles of ibuprofen is equal to 5.66 × 10²⁴.
What is a mole?A mole of a substance can be described as an international standard unit that is used to calculate a given count of any particles. The particles that are counted in the unit of the mole are as chemically identical entities, individually distinct.
A mole can be used to calculate atoms, molecules, ions, or other particular particles. The 6.023 × 10 ²³ number of units present in one mole is known as Avogadro’s constant.
Given, the number of moles of ibuprofen = 9.4 mol
The number of molecules of ibuprofen in one mole = 6.023 × 10²³
The number of molecules of ibuprofen in 9.4 moles = 6.023 × 10²³ ×9.4
Number of molcules of ibuprofen = 5.66 × 10²⁴ molecules
Learn more about the mole, here:
brainly.com/question/26416088
#SPJ1
Why do we calculate half-life?
Answers
Because it tells them about the amount of radiation that a given substance will give off.
The definition of radiation is energy that emanates from a source and moves at the speed of light through space. This energy has wave-like characteristics and is surrounded by an electric and magnetic field. Electromagnetic waves are another name for radiation.
Spectrum of electromagnetic waves shown In nature, there are many different types of electromagnetic radiation. One example is visible light.
The highest energy radiation comprises x-rays, gamma rays, and ultraviolet light.
Both X-rays and gamma rays are extremely energetic. They can take electrons out of atoms when they engage with them, ionizing the atom in the process. The four types of ionizing radiation that radioactive atoms can emit are alpha particles, beta particles, gamma rays, and neutrons.
Learn more about radiation here:
https://brainly.com/question/13934832
#SPJ4
if the density of your unknown liquid is 0.65 g/ml, calculate the volume in liters that 3 ml of your unknown liquid would occupy when vaporized at the temperature of your boiling water. Should 3 ml have been sufficient liquid to use in your experiment? Why or why not?
Answers
If the density of your unknown liquid is 0.65 g/ml, calculate the volume in liters that 3 ml of your unknown liquid would occupy when vaporized at the temperature of your boiling water.
The 3 ml liquid should be sufficient for a relatively low molecular weight liquid.
boiling point of water at 1 atm = 100 oC = 100 + 273 = 373 K
standard STP temperature = 273 K
STP volume = 22.4 L
Temperature = (373/273) = 1.37
density = 0.65 g/ml
Volume of 3 ml liquid when evaporated
= ((3 x 0.65)g/molar mass x 22.4 x 1.37
So knowing molar mass we can easily calculate the volume occupied by 3 x 0.65 g of liquid in vapor phase.
Temperature performs a vital function in-hospital treatment (both humans and animals), food, beverages, and agriculture. Our average fitness is regularly reliant upon the temperature in lots of methods as nicely.
The SI unit of temperature as consistent with the global system of devices is Kelvin.
Learn more about Temperature here:- https://brainly.com/question/2339046
#SPJ4
100 POINTS HELP WILL GIVE BRAINLIEST
Answers
Answer: 20 C
Explanation:
I would put it in 20 C because it is closer to the 20 C than the 30 C (it is below 25 C).
.how many moles are in 100.0g of CuF2
Answers
Answer:
0.985
Explanation:
moles= mass/Mr
Mr is the molecular mass
Cu=63.5
F2=38
Mr=63.5+38=101.5
moles=100/101.5=0.985
round answer if needed
there’s nothing to see here
Answers
Answer: Theres nothing to see here
Explanation: nothing at all,
How do you identify the charge of each element in a reaction?
Answers
To identify the charge of each element in a reaction, an atom becomes more stable, valence electrons are either simply taken up or released.
The periodic table helps us understand how different chemical elements interact with each other. As they become more stable, atoms either gain or lose valence electrons.
Nonmetallic elements gain electrons to produce negatively charged ions, while metals lose electrons to form positively charged ions. Metals (located on the left of the periodic table) will be positive, while non-metals (on the right) will be negative.
The particular ionic charge elements, however, must be known in order to identify the charges. The periodic table is also helpful when figuring out the electron configurations of atoms.
So, Atoms either simply take up or lose valence electrons as they becoming more stable.
To learn more about Identify the charge, Here :
https://brainly.com/question/11690635?referrer=searchResults
#SPJ4
problem 4.18 an alternating copolymer is known to have a number-average molecular weight of 100,000 g/mol and a degree of polymerization of 2210. if one of the repeat units is ethylene, which of styrene, propylene, tetrafluoroethylene, and vinyl chloride is the other repeat unit? why?
Answers
The repeating units in an alternating copolymer is known to have a number- average molecular weight of 100,000 g/mol is styrene.
If the other is Styrene, an alternating copolymer repeat unit forms. The formula to determine m is:
m = Mn / Dp
Styrene and unknown repetition units are the chain fractions of the alternating copolymer with the formula m = 100,000 / 2210 = 45.25g/mol.
Organic substance styrene has the chemical formula C6H5CH=CH2. This benzene derivative is an oily, colorless liquid, though older samples sometimes have a yellowish tint. The substance is easily vaporized and has a sweet smell, albeit it smells less good at higher concentrations.
For more information on polymers kindly visit to
https://brainly.com/question/17354715
#SPJ4
computational chemistry calculates physical and chemical properties of compounds based upon _________________.
Answers
Computational chemistry calculates the physical and chemical properties of compounds based on Computational chemistry
A chemical property is a property of a particular substance that can be observed in a chemical reaction. Some important chemical properties include flammability, toxicity, the heat of combustion, pH, radioactive decay rate, and chemical stability.
A chemical property is a property of a substance that becomes apparent during or after a chemical reaction. That are qualities that can only be ascertained by altering the chemical identity of a substance.
Physical properties of matter include color, hardness, malleability, solubility, electrical conductivity, density, melting point, and boiling point. These include reactivity, flammability, and the ability to rust. Reactivity is the ability of a substance to react chemically with another substance. Flammability is the ability of a substance to burn.
Learn more about chemical properties here:-https://brainly.com/question/2020832
#SPJ4
aluminum hydroxide reacts with nitric acid to form aluminum nitrate and water. what mass of water can be formed by the reaction of 15.0 g of aluminum hydroxide with excess nitric acid? a) 1.15 gb) 3.46 g c) 45.0 g d) 6.14 g e) 10.4 g
Answers
10.4 g of water can be formed by the reaction of 15.0 g of aluminum hydroxide with excess nitric acid.
Define mass.
It is the most basic component of matter and one of the fundamental quantities in physics. Mass is a term used to describe how much matter is there in a body. The kilogram (kg) is the SI unit of mass.
Al(OH)3 will react with HNO3 to form Al(NO3)3 and H2O
3Al(OH)3 + 3HNO3 → Al(NO3)3 + 3H2O
0.19229 × 3 = 0.57687 mol of H2O
Mass of H2O = mol×MW
= 0.57687×18
= 10.38366 g of H2O
Hence, 10.4g of water is formed.
Therefore, 10.4 g of water can be formed by the reaction of 15.0 g of aluminum hydroxide with excess nitric acid.
To learn more about mass from the given link.
https://brainly.com/question/29774205
#SPJ4
in a laboratory experiment, a 10.0 ml sample of sodium chloride solution is poured into an evaporating dish with a mass of 24.10 g. the combined mass of the evaporating dish and sodium chloride solution is 36.15 g. after heating, the evaporating dish and dry sodium chloride have a combined mass of 25.50 g. what is the molarity of the sodium chloride solution?
Answers
The molarity of the given solution was about 2.40 M.
What exactly is molarity?
The quantity of a material in a given volume of solution is measured in molarity (M). Molarity is defined as the number of moles of a solute in one litre of solution. A solution's molarity is also known as its molar concentration.
M is equal to n divided by v.
M stands for molar concentration or molarity.
n = number of moles of solute
v = solution litres
Sodium chloride salt mass = combined mass of evaporating with sodium chloride soution after heating minus mass of evaporating dish
=> 25.50g - 24.10g = 1.40g
1.40g sodium chloride salt mass
M= the number of moles of sodium chloride divided by the volume of solution
=>M = 0.023956 / 0.01
=>M = 2.40 M
To learn more about Molarity follow the given link: https://brainly.com/question/23243759
#SPJ4
The molarity of the given solution was about 2.40 M.
What exactly is molarity?
The quantity of a material in a given volume of solution is measured in molarity (M). Molarity is defined as the number of moles of a solute in one litre of solution. A solution's molarity is also known as its molar concentration.
M is equal to n divided by v.
M stands for molar concentration or molarity.
n = number of moles of solute
v = solution litres
Sodium chloride salt mass = combined mass of evaporating with sodium chloride soution after heating minus mass of evaporating dish
=> 25.50g - 24.10g = 1.40g
1.40g sodium chloride salt mass
M= the number of moles of sodium chloride divided by the volume of solution
=>M = 0.023956 / 0.01
=>M = 2.40 M
To know more about Molarity visit;
https://brainly.com/question/8732513
#SPJ4
4. how would your experimentally determined vinegar concentration have differed if you had over-titrated (added drops of naoh to the analyte beyond the stoichiometric equivalence point)?
Answers
The obtained percentage composition of acetic acid would be inaccurate and higher as greater volume of NaOH was being used in the experiment.
When a significant color change is seen, that marks the titration's endpoint (longer than 30 seconds). An excessive amount of titrant can cause the endpoint to be exceeded.
It would be an excess and the color of the solution in the flask would get significantly darker if a little bit more NaOH solution was added. Care must be made to avoid overshooting the terminus because it emerges unexpectedly. A last volume is taken from the buret after the titration has reached its endpoint.
Too much NaOH has been added if you exceed the endpoint and the solution turns bright pink. Add one or two drops of HCl to back-titrate the color to a lighter pink. The volumes consumed should be recorded.
To know more about titration, visit:
https://brainly.com/question/186765
#SPJ4
Which of the following molecules would have the highest vapor pressure at room
temperature?
CHCI3
CH₂Cl₂
CH3CI
CCI4
CH4
Answers
The molecule that would have the highest vapor pressure at room
temperature is CCl₄ because of its stronger intermolecular bonds and higher boiling point.
The correct option is D.
What is the vapor pressure of a substance?The vapor pressure of a substance is a measure of the tendency of a substance to change to a gaseous state.
The vapor pressure of a substance increases with an increase in temperature.
Also, the stronger the bonds that are present within the molecules of the substance, the higher will be the vapor pressure.
The vapor pressure of a substance is also related to the boiling point of that substance. The higher the boiling point of a substance, the higher the vapor pressure of that substance.
Learn more about vapor pressure at: https://brainly.com/question/2272852
#SPJ1