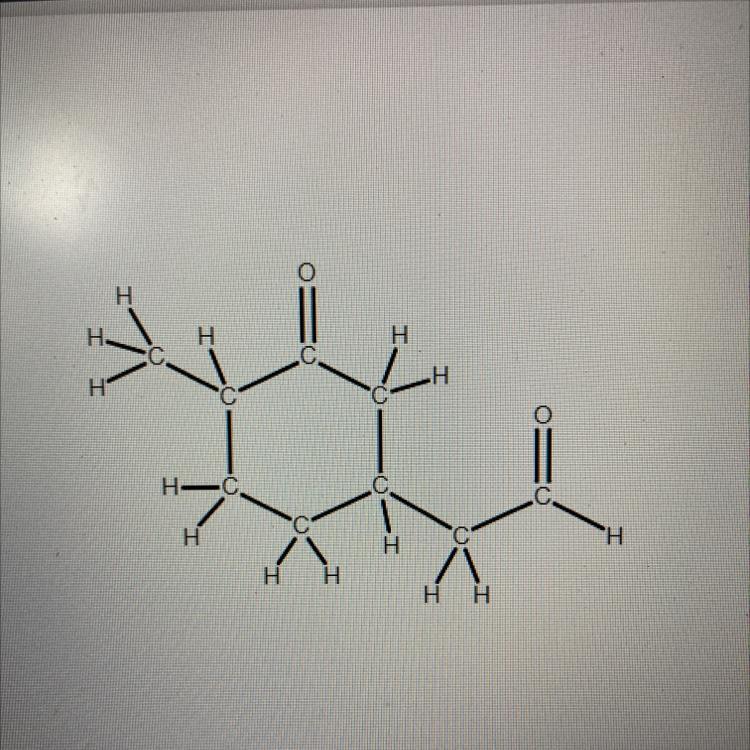
Answers
Let's look at the aldehyde. We must look for a similar structure for your task.
Circle the carbon on the right.
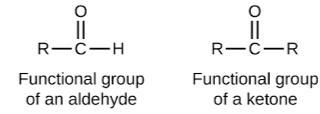
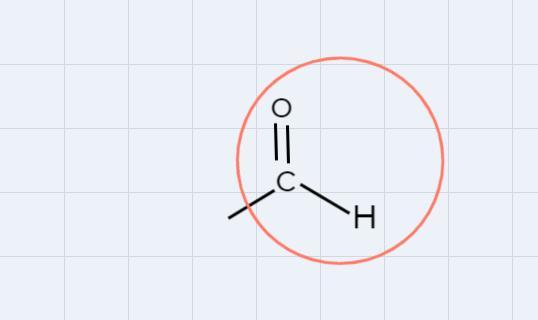
Related Questions
If a solution contained a substance of 4.67kg (molar mass of 34.6 g/mol) in 350mL, what is the molarity of the solution?
Answers
The molarity of a solution is the molar concentration of it.
It is calculated by:
[tex]M=\frac{n}{V}[/tex]Where n is the number of moles of the substance in the solution and V is the total volume of the solution.
The volume was given:
[tex]V=350mL=0.350L[/tex]And the number of moles we can calculate using the given mass and the molar mass:
[tex]\begin{gathered} m=4.67\operatorname{kg}=4670g \\ MM=34.6g/mol \end{gathered}[/tex]So:
[tex]\begin{gathered} MM=\frac{m}{n} \\ n=\frac{m}{MM}=\frac{4670g}{34.6g/mol}=134.97\ldots mol \end{gathered}[/tex][tex]M=\frac{134.97\ldots mol}{0.350L}=385.63\ldots mol/L\approx386mol/L[/tex]Thus, the molarity is approximately 386 mol/L.
Use but-2-ene to illustrate the difference between e and z isomers
Answers
The but-2-ene is a molecule with four carbons and the middle bond, (the second one) is a double-bond.
So, its structure is H₃C-CH=CH-CH₃.
However, the double bond makes two possible isomers, the "e" and "z":
The double bond, differently from the single bond, does not rotate, so these structures are not the same.
In the left one, the carbon groups have higher priority, and they are in alternating sides, bottom and top, and so it is called the E isomer.
In the right one, the carbon groups are on the same side, so it is called the Z isomer.
One of the reactions used to inflate automobile airbags involves sodium azide (NaN3): 2NaN3(s) ⇨ 2Na(s) + 3N2(g)Determine the mass of N2 produced from the decomposition of NaN3 shown at right
Answers
Step 1
The reaction provided:
2 NaN3 (s) ⇨ 2 Na (s) + 3 N2 (g) (completed and balanced)
---------------------
Step 2
Information provided:
100.0 g NaN3 decomposes
-----
Information needed:
The molar masses of:
NaN3) 65.00 g/mol
N2) 28.00
(please, the periodic table is useful here)
---------------------
Step 3
Procedure
By stoichiometry,
2 NaN3 (s) ⇨ 2 Na (s) + 3 N2 (g)
2 x 65.00 g NaN3 ----------------- 3 x 28.00 g N2
100.0 g NaN3 ----------------- X
X = 100.0 g NaN3 x 3 x 28.00 g N2/2 x 65.00 g NaN3
X = 64.62 g
Answer: 64.62 g of N2 produced
A slime mold is one of the slowest-moving organisms, traveling at top speeds of 1.02 millimeters per hour. What is the speed of a slime mold in inches per day?
Answers
We are given a speed of 1.02 mm/hr and we have to convert that speed into inches/day. To find the answer to our problem we can use a 2 steps conversion. First we can convert mm into inches and then the hr into days.
We know that there are 25.4 mm in 1 inch. So let's convert it.
25.4 mm = 1 inch
Speed = 1.02 mm/hr * 1 inch / (25.4 mm) = 0.04016 inch/hr
Speed = 0.04016 inch/hr
Then we know that there are 24 hours in 1 day.
24 hrs = 1 day
Speed = 0.04016 inch/hr * 24 hr/(1 day) = 0.964 inch/day
Speed = 0.964 inch/day
The speed of a slime mold in inches per day is 0.964.
I have tried initiating the question but I am not sure how to continue or what to do after moving things to the other side
Answers
+828kJ
Explanations:Given the following chemical reaction with their heat of reactions
[tex]\begin{gathered} 2Al(s)+\frac{3}{2}O_2(g)\rightarrow Al_2O_3(s);\text{ }\triangle H=-1675.7kJ..............\text{ 1} \\ CO(g)+\frac{1}{2}O_2(g)\rightarrow CO_2(g);\text{ }\triangle H=-282.7kJ...................2 \end{gathered}[/tex]In order to arrive at the resulting reaction, we will carry out the followiing transformation;
• Swap the reactant with product, of equation 1 and;
,• Multiply equation 2 by 3, to have;
[tex]\begin{gathered} Al_2O_3(s)\rightarrow2Al(s)+\frac{3}{2}O_2(g);\text{ }\triangle H=+1675.7kJ \\ 3CO(g)+\frac{3}{2}O_2(g)\rightarrow3CO_2(g);\text{ }\triangle H=-3(282.7)=-848.1kJ \end{gathered}[/tex]Cancel out the Oxygen element in both equation and add to have;
[tex]Al_2O_3(s)+3CO(g)\rightarrow2Al(s)+3CO_2(g);\text{ }\triangle H_{rxn}=+1675.7-848.1=+827.6kJ[/tex]Hence the enthalpy change of the reaction to 3sf is +828kJ
A chemist prepares a solution of copper(II) sulfate CuSO4 by measuring out 27.μmol of copper(II) sulfate into a 300.mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's copper(II) sulfate solution. Round your answer to 2 significant digits.
Answers
Step 1: Identify the given and do necessary conversions.
• 27.mol of copper ,
we know that 1 = 1*10^-6 mole ,
So, 27.mol*1*10^-6 = 27*10 ^-6 mole of copper.
• Volume of the solution = 300ml ,
300ml/1000ml = 0.3L
Step 2: Calculate the concentration in mol/L
Molarity( M /mole/L) = moles of the solute / volume
=27*10 ^-6 mole/ 0.3 L
=0.00009mol/L
• Therefore ,Concentraion of copper(II) sulfate =9.0x10^-5mol/L
( 2 significant digits)
Which property suggests that magnesium is a transition metal? A. It burns in oxygen to form the oxide mno2, B. It forms coloured compunds?, c. It has only one isotope,D. It reacts with dilute hydrochloric acid to give hydrogen
Answers
Answer:
D
Explanation:
as a transition metal, when put in dilute hydrochloric acid it will bubble vigorously and produce hydrogen gas
Determine the pOH of a solution that has a [H+] of 0.0059
Answers
answer and explanation
the sum of pH and pOH is always equal too 14
and so if we apply that principle we have the following
pOH + pH = 14
and we are told that the pH is 0.0059 so we substitute this in the equation
pOH +0.0059 = 14
pOH = 14 - 0.0059
pOH = 13.99
Match the reaction to the type that best describes it.NaF + KBr →NaBr + KF?CombinationMg + MnCl2 →Mn + MgCl2?Decomposition2HCl → H2 + Cl2?Single replacementaw2Na + O2 →Na202?Double replacement
Answers
Explanation
Let us first define the different types of reaction.
A combination reaction is one in which two reactants combine to form a single product.
[tex]2Na+O_2\rightarrow Na_2O_2[/tex]A decomposition reaction will be one in which a reactant breaks down into two or more products.
[tex]2HCl\rightarrow H_2+Cl_2[/tex]A simple displacement reaction will be one in which a reagent, which is in its natural state, displaces one element bound to another. As a product, we will have the previously linked element now in its free form.
[tex]Mg+MnCl_2\rightarrow Mn+MgCl_2[/tex]In a double displacement reaction two linked elements move past each other and take each other's place.
[tex]NaF+KBr\rightarrow NaBr+KF[/tex]How has computer modeling made weather
predictions more accurate?
Answers
Answer:
Smaller grid spacing closes the gap on observations in time and space, resulting in higher resolution model output that is more accurate and reliable.
A solution is formed by dissolving 25.0 g of NaCl in 750.0 grams of H2O.What is the mass of solute? Group of answer choices25.0 g750.0 g775.0 g
Answers
1) Solute. A solute is a substance that dissolves when put into another substance (the solvent).
NaCl dissolves when placed into water. So, NaCl is the solute.
The mass of the solute is 25.0 g.
.
Tin forms an ion with a 2+ charge. What is the Electron configuration of this ion?
Answers
STEP-BY-STEP EXPLANATION
What to find? The electronic configuration of tin ion
Tin is an element with an atomic number of 50.
NB: Atomic number = number of proton
Hence, the number of proton of tin = 50
[tex]Sn^{2+}[/tex]The above tin ion means it has lost two electrons, which make it has a total electron of 48
To write the electronic configuration of tin ion, we will be using the SPDF Notation
spdf are names given to orbitals that hold electrons in atoms.
s - orbital
p- orbital
d- orbital
f -orbital
[tex]\begin{gathered} \text{Electronic configuration of Sn}^{2+}\text{ is written below} \\ 1s^22s^22p^63s^23p^64s^23d^{10}4p^65s^24d^{10} \end{gathered}[/tex]The Apollo 13 mission was determined to be a “successful failure”. Although the astronauts did not land on the Moon, they were successfully brought home. A thorough investigation of the explosion, based on records of manufacturing processes and maintenance logs, tracked the failure of the oxygen tank to multiple faults. None of the problems, had they occurred alone, would have been critical, but together they led to a near disaster for the crew of Apollo 13. How much carbon dioxide would have been removed from the breathing environment if the scrubber reaction produced 50.0 kilograms of lithium carbonate? Use dimensional analysis.
Answers
29,769.95g of carbon dioxide can be removed.
1st) It is necessary to write the balanced chemical reaction:
[tex]2\text{LiOH + CO}_2\text{ }\rightarrow Li_2CO_3+H_2O[/tex]According to the balanced equation, 1 mole of carbon dioxide (CO2) can be removed by producing 1 mole of lithium carbonate (Li2CO3).
Using the molar mass of carbon dioxide and lithium carbonate we can convert moles into grams:
- CO2 molar mass: 44 g/mol
- Li2CO3 molar mass: 73.9 g/mol
With the molar mass we can see that 44g of carbon dioxide (1 mole) can be removed by producing 73.9 g of lithium carbonate.
2nd) Now we can calculate the amount of carbon dioxide that can be removed from the breathing environment if the scrubber reaction produces 50.0 Kg of Li2CO3, using a mathematical rule of three:
[tex]\begin{gathered} 0.0739kgLi_2CO_3-44gCO_2 \\ 50kgLi_2CO_3-x=\frac{50kgLi_2CO_3\cdot44gCO_2}{0.0739kgLi_2CO_3} \\ x=29,769.95gCO_2 \end{gathered}[/tex]Here, it is important to convert the grams of Li2CO3 to kg before doing the calculus.
So, 29,769.95g (29.8kg) of carbon dioxide can be removed.
ch3)2c(oh)ch2ch3 skeletal structure, Functional groups, compound type & alcohol class if it’s an alcohol
Answers
The skeletal structure of an organic compound is an abbreviated representation of its molecular structure, they are quick and easy to draw.
For example, the following image shows the skeletal structure of a compound:
The peaks represent the carbons. We must remember that carbon can have a maximum of 4 bonds.
Now, I will show you how is the structure of this specific compound:
This is ternary alcohol, called 2-methyl-2-butanol. If you see carefully, you will notice that each carbon has 4 bonds. The functional groups present will be OH. The skeletal structure will be:
Describe the energy transformations in each:1. When the flashlight is turned on, a flow of electrons is created by the batteries. The electricity passes through the incandescent lightbulb, causing the tungsten filament in the lightbulb to heat up. When the metal is hot enough, photons of light are emitted.
Answers
First, it is the chemical energy because of the batteries, then this energy is transforming to electrical because of the flowing of electrons. Before this, the electrical energy is going to be light energy for the light bulb and finally, we have the heat energy of the light bulb. The order is:
[tex]\text{Chemical}\to electricity\to light\to heat.[/tex]Solutions of potassium iodide and lead (II) are mixed. The resulting lead compound is a precipitate.a) substitute symbols and formulas for words b) predict the products. If no reaction occurs, write "NR" after the yields sign. c) include abbreviations for the physical state d) balance the equation
Answers
The two compounds that react are:
Potassium iodide: KI
Lead (II):
[tex]Pb^{+2}[/tex]When the two solutions are mixed the following reaction takes place:
[tex]2KI(aq)+Pb^{+2}(aq)\text{ }\rightarrow2K^{+1}(aq)+PbI_2\text{ (s)}[/tex]The resulting product is called lead (II) Iodide.
Patrick is a 16 year old boy whose body has stopped producing osteoclasts. What does this mean for his bones? What other parts of
his body will be affected by this?
Answers
Answer:
Osteoclasts are the only bone resorbing cells, so, when his body stops producing osteoclasts, it results in deficiency of bone turnover and In osteopetrotic-like diseases.The bone will be affected by this.
How does the force that holds ions together explain the properties of an ionic compound?
Answers
There are a lot of properties of ionic compounds to explain.
The most characteristic ones are:
- High melting points
- Hard and brittle
- Electrical conductivity
All of these can be explained by the forces in ionic compounds.
The forces that maintain the ions together are electrostatic forces, which come from the charged ions with opposite signs.
Electrostatic forces are very strong, so and in ions this occur in a crystal lattice, so we need a lot of energy to break this attraction. Because of that, we need a high temperature to make it has enough energy to break this attraction and melt.
This strong attraction also makes the ionic compounds hard to break, because we will need a lot of force to break the ions appart. However, once enough force is applied, its lattice structure is shifted and ions with same charge becomes close to each other, making a repulsive force that breaks them appart, giving them the brittle property.
An electrical conductivity happens when there are free charges in a system. The ionic compounds are composed by charged particles, so if they are free to move, they will conduct electricity. So, when the compound is melted of dissolved, the charged ions, that once were together by the electrostatic force, now can conduct electricity.I have finished answering the question.
Calculate the [H3O+] of the following solutions: ph = 11.8
Answers
Answer:
The [H3O+] is 1.58x10-12 M.
Explanation:
To calculate the [H3O+] from the pH, we can use the pH formula and replace the value of pH:
[tex]\begin{gathered} pH=-log{}\lbrack H_3O^+\rbrack \\ 11.8=-log\lbrack H_3O^+\rbrack \\ 10^{(-11.8)}=\lbrack H_3O^+\rbrack \\ 1.58*10^{-12}=\lbrack H_3O^+\rbrack \end{gathered}[/tex]So, the [H3O+] is 1.58x10-12 M.
When copper is heated with an excess of sulfur, copper(I) sulfide is formed. In a given experiment, 0.0970 moles of copper was heated with excess sulfur to yield 2.37 g copper(I) sulfide. What is the percent yield?
Answers
Answer: the percent yield of the reaction is 30.7%
Explanation:
The question requires us to determine the percent yield of the reaction that produces copper(I) sulfide, given the theoretical yield and actual yield.
The following information was provided by the question:
Amount of Cu used = 0.0970 mol of Cu
Actual yield of reaction = 2.37 g of Cu2S
To solve this problem, we'll need to determine the theoretical yield of the reaction, considering the amount of copper (Cu) used and the balanced chemical equation for this reaction, and then calculate the percent yield of the reaction.
1) Determining the theoretical yield of reaction:
The reaction between copper and sulfur to produce copper (II) sulfide can be written as:
[tex]2Cu\text{ + S}\rightarrow Cu_2S[/tex]Considering that 0.0970 moles of Cu were used in excess of S, and the stoichiometry of the reaction, we can calculate the amount of Cu2S produced:
2 mol Cu ---------------------- 1 mol Cu2S
0.0970 mol Cu ------------ x
Solving for x, we have that 0.0485 moles of Cu2S should be obtained from the reaction.
2) Calculating the percent yield of the reaction:
The percent yield of a chemical reaction can be calculated using the following equation:
[tex]percent\text{ yield = }\frac{theoretical\text{ yield}}{actual\text{ yield}}\times100\%[/tex]Note that the theoretical and actual yield values must be used in the same units. Thus, we need to convert the mass given for actual yield (2.37g) to number of moles, considering the molar mass of copper (I) sulfide (159.16 g/mol):
[tex]\begin{gathered} number\text{ of moles = }\frac{mass\text{ of sample \lparen g\rparen}}{molar\text{ mass \lparen g/mol\rparen}} \\ \\ number\text{ of moles = }\frac{2.37g}{159.16g/mol}=0.0149\text{ mol} \end{gathered}[/tex]Therefore, the actual yield corresponds to 0.0149 moles of Cu2S.
Now, we can calculate the percent yield of the reaction:
[tex]percent\text{ yield = }\frac{0.0149\text{ mol}}{0.0485\text{ mol}}\times100\%=30.7\%[/tex]Therefore, the percent yield of the reaction is 30.7%.
Why does the ice pack get colder when the inside barrier is broken and the chemicals mix together?
Answers
The ice pack gets colder when the inside barrier is broken and the chemicals mix together as instant cold packs employ this type of endothermic reaction.
Endothermic reactions are those that involve the absorption of heat.When the chemical ammonium nitrate is dissolved in water, the resultant solution is colder than either of the starting ingredients. This is an illustration of an endothermic process. Instant cold packs employ this type of endothermic reaction.Water is the substance that fills the cold pack. Another plastic bag or tube carrying fertilizer ammonium nitrate is submerged in the water. The tube is broken when you strike the cold pack, allowing the water and fertilizer to mix. The endothermic reaction this mixture produces results in heat absorption.
To learn more about endothermic reaction visit:
brainly.com/question/23184814
#SPJ9
Balance these chemical equations. (Use the lowest possible whole number coefficients.)Fe + O2 → FeO
Answers
Chemistry =>Chemical Reactions => Balancing equations
We have the oxidation of iron in the equation.
To balance the equation we must start by counting the atoms of each element on both sides of the reaction.
We will balance the oxygen. We have 2 atoms of oxygen on the reactants. So we write coefficient 2 on the FeO molecule.
[tex]Fe+O_2\rightarrow2FeO[/tex]Now, we balance the iron. We have 2 Fe atoms, so we write the coefficient 2 on the Fe molecule. We have:
[tex]2Fe+O_2\rightarrow2FeO[/tex]Now, the equation of the reaction is balanced.
Answer: 2Fe + O2 →2FeO
What are three characteristics of nonmetals?
Answers
Characteristics of nonmetals
1. What characterizes non-metals is that they lack the ability to conduct electricity, therefore they are poor conductors, unlike metals.
2. Non-metallic compounds in general are the basis for forming organic compounds essential for life.
3. Non-metal elements do not reflect light, are non-corrosive and are brittle, and break easily.
4. They also have high electronegativity, so when they form an ionic bond they tend to take electrons.
Determine the pH of a 0.536002 M C6H5CO2H M solution if the Ka of C6H5CO2H is 6.5 x 10-5. Record your answer to two decimal places
Answers
To solve this problem, we could use the ICE table as follows:
First of all, the reaction that we're working with is:
[tex]C_6H_5CO_2H\leftrightarrow C_6H_5CO^-_2+H^+_{}[/tex]Now, let's make the table:
As you can notice, the initial concentration of C6H5CO2H is 0.536002M, and as the products haven't been formed yet, their concentration is 0.
When the change happen, the concentration of the reactant will decay "x" and the concentration of the products will increase "x". (That's the reason of the signs).
And finally, the equilibrium.
We also know that:
[tex]K_a=6.5\cdot10^{-5}[/tex]And, this equilibrium constant comes from the following:
[tex]K_a=6.5\cdot10^{-5}=\frac{\lbrack H^+\rbrack\lbrack C_6H_5CO^-_{2^{}}\rbrack}{\lbrack C_6H_5CO_2H\rbrack}[/tex]Where all concentrations are the equilibrium concentrations.
If we replace, we got that:
[tex]\begin{gathered} 6.5\cdot10^{-5}=\frac{\lbrack H^+\rbrack\lbrack C_6H_5CO^-_{2^{}}\rbrack}{\lbrack C_6H_5CO_2H\rbrack} \\ \\ 6.5\cdot10^{-5}=\frac{x\cdot x}{0.536002-x} \\ \\ 6.5\cdot10^{-5}=\frac{x^2}{0.536002-x} \end{gathered}[/tex]We're going to solve this equation for x:
[tex]\begin{gathered} 6.5\cdot10^{-5}(0.536002-x)=x^2 \\ =3.484013\cdot10^{-5}-6.5\cdot10^5x-x^2=0 \end{gathered}[/tex]And, using the quadratic formula we obtain that the value of x is 0.000587014 approximately.
Now, if x=0.000587014, that means that [H+] = 0.000587014
And finally, remember that the pH of a solution is defined as:
[tex]\begin{gathered} pH=-\log \lbrack H^+\text{\rbrack} \\ pH=-\log \lbrack0.000587014\rbrack \\ pH=3.23 \end{gathered}[/tex]Therefore, the pH is 3.23.
What is the molality of a solution containing 10.0 g Na»SO dissolvedin 1000.0 g of water?
Answers
Given Data
mass of solute Na 10.0 g
mass of solvent 1000.0g water
to calculate:
molality of solution
molalilty is defined as the mols of a solute divided by the Kg of the solvent
therefore mols of Na = mass/ molar mass
= 10.0g/22.98g/mol
= 0.44 mols
1 Kg = 10000. grams
Therefore molality of solution
= 0.44 mols/ 1 Kg
= 0.44 mols/Kg
Research: Find 4 strategies that are used to prevent rusting of car parts and/or water pipes? Briefly explain how they work in your own words. What are some pros and cons of these methods?
Answers
Question
Research: Find 4 strategies that are used to prevent rusting of car parts and/or water pipes? Briefly explain how they work in your own words. What are some pros and cons of these methods?
Answer
Method 1: Applying Oil - Coating materials with oil will help to prevent rust or slow its formation since it prevents moisture from reaching the iron, which can get oxidized.
Pros: It can be applied easily to most materials and it's of relatively low cost
Cons: Some materials can't be covered with oil because they can interfere with the process they are designed for. Example: Food transport machinery
Method 2: Paint the metal - Similarly to the oil a good amount of paint can cover the metallic parts and prevent moisture from the air to get in direct contact with the iron.
Pros: It is easy to apply paint to most metals.
Cons: Contrarily to oil, paint can be more expensive depending on the application.
Method 3: Galvanization - is the process of applying a protective zinc coating to iron or steel materials to prevent or slow the rusting processes.
Pros: The galvanization process makes steel highly durable and the material usually becomes scratch free.
Cons: Steel structures that are either too big or too small are unsuitable for the galvanization process.
Method 4: Store in a controlled environment - Metals corrode easily when exposed to environments with high humidity. To preserve a metal in good condition, it should be stored in environments with low humidity content and not in contact with salts or acids.
Pros: It does not alter the properties of the equipment.
Cons: Not all metal objects can be stored in environments containing low humidity.
What is the name of the compound P4Cl7?
1. phosphorus(IV) heptachloride
2. phosphorus hexachloride
3. potassium(IV) chloride
4. tetraphosphorus heptachloride
5. quattrophosphorus sevenchloride
6. tetraphosphorus chlorate
Answers
4
beacause it is4 is teta and 7 is hepta
How many moles of oxygen will be needed to combust 20.50 moles of C7H16?
Answers
Answer
225.5 mol O₂
Procedure
Assuming the complete combustion of heptane, we will consider the following reaction for our calculations.
C₇H₁₆ + 11 O₂ → 7 CO₂ + 8 H₂O
The moles of oxygen can be determined by stoichiometry
[tex]20.50\text{ mol C}_7\text{H}_{16}\frac{11\text{ mol O}_2}{1\text{ mol C}_7\text{ H}_{16}}=225.5\text{ mol O}_2[/tex]Describe a real-world example that demonstrates this relationship. In your description identify the two variables (temperature and volume) and describe how one changes as the other is increased or decreased.
Answers
A real-world example that can demonstrate the relationship between the variables temperature and volume is, for example, when we inflate a balloon for a party.
- First, we fill the ballon with a gas (air).
- Second, if we tie the balloon and heat it with some source of heat, we will see that the ballon begins to increase in volume.
So, as we increase the temperature of the gas inside the ballon, the balloon increases in volume.
This happens because temperature and volume are variables directly proportional through the Ideal Gas Law, whose formula is:
[tex]P\mathrm{}V=n\mathrm{}R\mathrm{}T[/tex]In the formula:
P is the pressure of the gas.
V is the volume of the gas.
n is the number of moles.
R is the constant of the gases.
T is the temperature of the gas.
what is the pH of a HCOOH solution that is 0.018m?
Answers
Answer:
[tex]pH\text{ = }2.75[/tex]Explanation:
Here, we want to get the pH of the given molecule
Firstly,we write the ionization equation so as to get the number of hydrogen atoms from its dissociation
We have this as:
[tex]HCOOH_{(aq)}+H_2O_{(l)}\rightleftarrows HCOO^-_{(aq)}+H_3O^+_{(aq)}[/tex]The Pka value for formic acid is 3.75
We have it that:
[tex]\begin{gathered} K_a=10^{-pKa} \\ K_a=10^{-3.75}\text{ = }1.78\text{ }\times10^{-4} \end{gathered}[/tex]Using the ICE equation for the equilibrium constant,we have it that:
[tex]K_a\text{ = }\frac{\lbrack HCOO^-\rbrack\lbrack H_3O^+\rbrack}{\lbrack HCOOH\rbrack\rbrack}[/tex]Thus, we have:
[tex]0.000178=\frac{x^2}{0.018\text{ - x}}^{}[/tex]Since x is very small as opposed to tthe initial acid concentration, we approximate x as 0 below
Thus,we have:
[tex]\begin{gathered} (0.018\times0.000178)=x^2 \\ x\text{ = }\sqrt[]{0.018\times0.000178} \\ x\text{ = 0.00179} \end{gathered}[/tex]Finally, we know that the pH is the negative logarithm to base 10 of the concentration of hydroxonium ion
[tex]\begin{gathered} pH=-log\lbrack H_3O^+\rbrack_{} \\ pH\text{ = -log\lbrack{}0.00179\rbrack} \\ pH\text{ = 2.75} \end{gathered}[/tex]