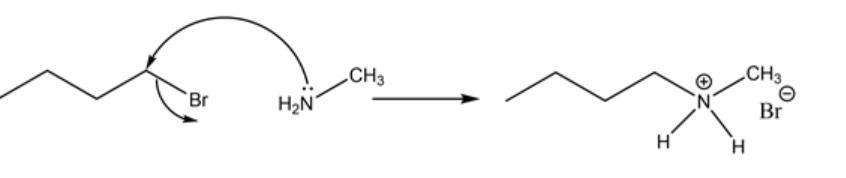
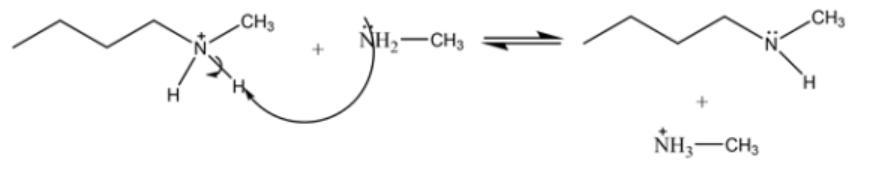
primary amines can be converted into secondary amines by reaction with haloalkanes. this reaction is difficult to achieve in the lab because of the large number of byproducts. select the possible products or byproducts of the reaction.
Answers
primary amines can be converted into secondary amines by reaction with haloalkanes. this reaction is difficult to achieve in the lab because of the large number of byproducts. select the possible products or byproducts of the reaction.
The concept used to solve this problem is the knowledge of nucleophilic substitution reactions.
The Sn2 reaction or the bimolecular nucleophilic substitution reaction is a concerted type of reaction followed by the trigonal bipyramidal-shaped transition state which results in the formation of the product of inverted stereochemistry.
Fundamentals
Nucleophilic substitution reaction involves the primary attack of a nucleophile to the sigma square antibonding orbital of the carbon of alkyl halide, which in effect attach the nucleophile to that carbon and the removal of the halogen as a leaving group.
Attack of lone pair of nitrogen of substituted ammonia to the carbon-halogen bond of the halogen alkane. The initial attack is shown below.
Attack of the substituted ammonia acts as a base and abstracts proton from the alky ammonium halide salt to form the secondary amine. The attack of substituted ammonia is shown below
To know more about primary amines:
https://brainly.com/question/29589832
#SPJ4
Related Questions
A graduated cylinder is filled to
25.0 mL with water and a piece of a
red mineral is placed in the cylinder,
displacing the level to 35.0 mL.
What is the volume of the red
mineral piece in milliliters?
volume = [?] mL
Answers
The volume of the red mineral piece in millilitres is 10 millilitres.
How to calculate volume?Volume in chemistry is a measure of the amount of space that matter occupies.
Matter is defined as a physical substance that occupies space and has a mass. In physical sciences like chemistry, the standard unit of volume is cubic metres (m³). From this unit, other units are derived including litre (L) and millilitre (mL).
According to this question, a graduated cylinder is filled to 25.0 mL with water and a piece of a red mineral is placed in the cylinder, displacing the level to 35.0 mL.
This suggests that the volume of the red mineral placed in the cylinder can be calculated as follows:
Volume of red mineral = 35.0mL - 25.0mL = 10.0mL
Therefore, 10mL is the volume of the piece of red mineral.
Learn more about volume at: https://brainly.com/question/17035745
#SPJ1
How many covalent bonds can phosphorus form?
Answers
In the case of phosphorus, 5 covalent bonds are possible - as in PCl5.
A covalent bond is formed when two atoms exchange one or more pairs of electrons. The two atomic nuclei are concurrently drawing these electrons to them. When the difference between the electronegativities of two atoms is too tiny for an electron transfer to take place to create ions, a covalent bond is formed. Bonding electrons are collectively referred to as the electrons that are present between the two nuclei. The "glue" that holds the atoms in molecular units together is the bound pair. The simplest material with a covalent link is the hydrogen molecule.
To know more about covalent bonds, visit;
brainly.com/question/10777799
#SPJ4
What are 4 things to consider when storing chemicals?
Answers
The 4 things to consider when storing chemicals is flammable and oxidizing chemical store separately , label the chemical , volatile chemicals in ventilated area and chemicals should be placed in the closed containers.
The chemicals should be stored in the closed container to keep it safe. the flammable and the oxidizing chemical should be store separately because they are incompatible to each other. the chemical should be placed sperate from the any kind of the ignition source. the volatile chemicals should be placed in the ventilated cabinets.
Thus, it very important to store the chemicals very safely so that it can not be hazardous to anyone.
To learn more about chemicals here
https://brainly.com/question/13145357
#SPJ4
Nutritionists express energy in calories, which are in fact 1000 real calories. one real calorie is equivalent to 4.18 joules, the universal energy unit. thus, one nutrition calorie is equivalent to:____.
Answers
Nutritionists express energy in calories, which are in fact 1000 real calories. one real calorie is equivalent to 4.18 joules, the universal energy unit. thus, one nutrition calorie is equivalent to 4.18 kilojoules.
Joule is the fundamental energy unit of the metric system, or the International System of Units in a later, more thorough version (SI). In the end, the meter, kilogram, and second are used to characterize it.
Calorie (cal) (cal): In the past, the definition of a calorie included the heating of water. Consequently, according to a conventional definition, one calorie is the quantity of heat needed to raise the temperature of one gram of water by one degree Celsius, from 14.5 to 15.5 degrees.
The "calorie" measured for other temperature ranges differs slightly from this, which is frequently referred to as the 15 °C calorie. The calorie has more recently been defined in terms of the joule; historically, the calorie and joule have been equivalent to mechanical heat.
For more information on calorie kindly visit to
https://brainly.com/question/22374134
#SPJ4
100 POINTS WILL MARK BRAINLIEST HELP PLEASE
Answers
Answer: D
Explanation:
Assuming this is a diagram to show how different substances react with each other, each number is representing a different substance.
Therefore, for example, substance 2 reacts with substance 1 to create a new substance. Or substance 4 reacts with substance 3 to create a new substance.
You can think about it like this:
Orange + water --> orange juice
or
Apple + orange --> a rather depressing fruit salad
However, what do you get when you add water + water?
Water + water --> surprise... WATER!!!
Therefore between the same substances, there will no reaction.
Hoped this helped! :)
9. Calculate the percent error for a lab when the students determined the actual yield to be 66.89
theoretical yield is 74.65. SHOW ALL WORK, Box Final Answer!
Answers
Answer:11.60%
Explanation:
[tex]Percent Error = \frac{Actual - Theoretical}{Actual} (100)\\[/tex]
[tex]Percent Error = \frac{74.65-66.89}{66.89} (100) = 11.60%[/tex]%
to prepare a 200.0 ml solution of 5.0 m nacl, how many grams of nacl would be added to the volumetric flask?
Answers
To prepare a 200.0 ml aqueous solution of 5.0M NaCl, grams of nacl is 10g.
(w/v) % = 5
Volume = 200 mL
Hence, mass of NaCl=200×5/100 = 10g
What is an aqueous solution?
An aqueous solution is an answer in which the solvent is water. it's far commonly shown in chemical equations with the aid of appending (aq) to the relevant chemical system. for instance, an answer of desk salt, or sodium chloride (NaCl), in water could be represented as Na+(aq) + Cl−(aq). The word aqueous (which comes from aqua) way concerning, associated with, just like, or dissolved in, water. As water is an first-rate solvent and is also evidently ample, it's miles a ubiquitous solvent in chemistry. Because water is frequently used as the solvent in experiments, the word answer refers to an aqueous solution, unless the solvent is detailed.To know more about aqueous solutions, click the link given below:
https://brainly.com/question/14987339
#SPJ4
An olympic-size pool is 50. 0 m long and 25. 0 m wide. How many gallons of water (density = 1. 0 g/ml) are needed to fill the pool to an average depth of 4. 8 ft?.
Answers
To fill the 50 m long and 25 wide pool to an average depth of 4.8 ft., the needed volume of water is 483,101 gallons
Suppose we have a rectangular prism with length l, width w, and height h, then the volume of the prism is given by:
V = l x w x h
In the problem, the given parameters are:
l = 50 m
w = 25 m
h = 4.8 ft. = 1.463 m
Plug these parameters into the formula:
V = 50 x 25 x 1.463
V = 1,828.75 m³
Next we need to convert m³ to gallons
1 m³ = 264.17 gallons
Hence,
V = 1,828.75 m³ = 1,828.75 x 264.17 gallons
V = 483,101 gallons
Learn more about volume here:
brainly.com/question/17871570
#SPJ4
A 1200 kg car runs head on into a 2500 kg truck, both traveling at 12m/s
Answers
The common velocity of the car and the truck after the collision is 12 m/s
How do I determine the common velocity?From conservation of linear momentum, we understood that momentum before collision is equal to momentum after collision ie
Momentum before = Momentum after
m₁u₁ + m₂u₂ = v(m₁ + m₂)
Where
m₁u₁ are the mass and speed of the 1st object before collisionm₂u₂ are the mass and speed of the 2nd object before collisionv is the velocity of both object after collision.Using the above formula, we can obtain the common velocity of the car and truck. This is shown below:
Mass of car (m₁) = 1200 KgMass of truck (m₂) = 2500 KgVelocity of car (u₁) = 12Velocity of truck (u₂) = 12 m/sCommon velocity (v) = ?m₁u₁ + m₂u₂ = v(m₁ + m₂)
Divide both sides by (m₁ + m₂)
v = [m₁u₁ + m₂u₂] / (m₁ + m₂)
v = [(1200 × 12) + (2500 × 12)] / (1200 + 2500)
v = [14400 + 30000] / 3700
v = 44400 / 3700
v = 12 m/s
Thus, the common velocity is 12 m/s
Learn more about velocity:
https://brainly.com/question/14723857
https://brainly.com/question/20355178
#SPJ1
Complete question:
A 1200 kg car runs head on into a 2500 kg truck, both traveling at 12m/s. What is their common velocity after the collision?
- Predicting What is the product of the
synthesis reaction between magnesium
and iodine? Explain your answer.
—HELPPP
Answers
The reaction of Magnesium metal with Iodine will yield Magnesium Iodide. The reaction would be as follows:
Mg (solid) + I2 (gas) ---> MgI2 (solid)
The two valence electrons of Mg will be distributed to each of the Iodine atoms in I2 gas. In the end, Mg ended up being Oxidized because it lose two electrons and I2 is reduced as it gained electrons.
MgI2 is classified as an ionic salt and it is highly soluble in water.
100 POINTS BRAINLIEST PICTURE BELOW
Answers
Answer:
D
Explanation:
Sparking can be caused by defective cooktop elements, loose connectors, a wire with frayed insulation or a broken wire arcing over to the appliance metal frame, says Appliance Repair. If the cause of the sparking is a broken wire or a wire with frayed insulation, do not attempt to splice the wire; you must replace it. Repair Clinic suggests checking the spark ignition switches, as they might be defective and in need of replacing.
Answer:
A. If you got chemicals in your eye ...
Explanation:
You want to know the use of the safety equipment shown.
Eye washThe picture shows a eye-wash station. Its purpose is to flood your face and eyes with water in the event that you accidentally contaminate your eyes with foreign matter. You would use this equipment ...
If you got chemicals in your eye when a test tube broke.
Consider a galvanic cell with a beaker of Chromium(III) nitrate Cr(NO3)3 and a beaker of Copper(II) nitrate Cu(NO3)2. The Chromium(III) nitrate beaker contains a strip of chromium, and the Copper(II) nitrate cell contains a strip of copper. A wire runs between the strips. The reaction that occurs is as follows: 2Cr(s) + 3Cu2+(aq) → 2Cr3+(aq) + 3Cu(s) In three to five sentences, list which electrode is the anode and which is the cathode and the half reactions that occur at each electrode.
Answers
At the anode, the half reaction that takes place is seen as: 2Cr(s) → 2Cr³+ (aq) + 3e-.
At the cathode, the half reaction that takes place is 3Cu² (aq) + 3e- → 3Cu(s).
The two half reactions that fused to form the total reaction 2Cr(s) + 3Cu² (aq) → 2Cr³+ (aq) + 3Cu(s).
How is a galvanic cell put to use?Galvanic cells is one that make use of the electrical energy obtained by the transport of electrons during a redox reaction to carry out practical electrical work.
A galvanic cell or voltaic cell is an electrochemical device that transforms the chemical energy of spontaneous redox reactions into electrical energy. electrical cell A voltaic cell is an electrochemical device that produces electricity through chemical processes.
Therefore, note that in the above galvanic cell:
The strip of chromium in the Chromium(III) nitrate beaker - anodeThe strip of copper in the Copper(II) nitrate beaker - cathode.Learn more about galvanic cell from
https://brainly.com/question/15096829
#SPJ1
how is electronegativity used in determining the ionic and doric relevant character of the bonding between two elements
Answers
How polar a link will be depends on how differently electronegative two atoms are from one another. Since there is no variation in electronegativity in a diatomic molecule with two identical atoms, the bond is nonpolar or pure covalent.
What is polar and non polar?
Polarity is the term used to describe objects that differ at either end. Some molecules also contain positive and negative ends, and when this occurs, we refer to those molecules as being polar. We refer to someone as non-polar if they don't. Polar objects can be drawn to or repelled by one another (opposite charges attract, alike charges repel).
To learn more about polar and non polar
Here: https://brainly.com/question/545359
#SPJ4
What forces are in CH2O?
Answers
The formaldehyde CH₂O atom contain the forces of covalent bond.
The carbon atom has 4 valence electron.
It cannot directly gain or loose for electron that is why it shares the electrons with other atoms to form bonds with it.
In the formaldehyde CH₂O atom the carbon present at the center forms Bond with the oxygen and the hydrogen atom.
The hydrogen atom requires one electron each while the oxygen item requires to electrons for a stable electronic configuration.
When these of hydrogen and oxygen shares electrons with the central carbon atom they experience is a force created due to the covalent bonds.
To know more about covalent bond, visit
https://brainly.com/question/28366450
#SPJ4
What is the molarity of 40g of NaOH dissolved in 1.25 Litre of water?
Answers
0.8 M is the molarity of 40g of NaOH dissolved in 1.25 Litre of water
To find the molarity of solution we will use following expression ;
Molarity (M) = n/V(ml)×1000
Where, n = number of moles of solute
V = volume of solution in ml.
In the given problem,
NaOH is Solute in solution
Given mass of sodium hydroxide = 40 grams
Molar mass of NaOH = 40 g/mol
Total volume of the solution = 1.25 L
Number of moles of sodium hydroxide(n):-
n = mass of NaOH/molar mass of NaOH
n = 40g/(40g/mo)l
n = 1 mole of NaOH
Substitute the values of volume and number of moles of solution in the molarity formula,
Molarity (M) = 1mole/1.25 L
M = 0.8 M
Hence, The Molarity of NaOH is 0.8 M.
To know more about molarity visit here ; https://brainly.com/question/16587536?referrer=searchResults
#SPJ4
A sample of gas weighs 4.84 g and occupies a volume of 0.918 l at 35 °c and 850 torr. identify the gas sample.
a. NH3 (molar = Mass 17,03 B/moll
b. CHCI3 (molar mass 119 4 B/mol)
c. SO2 (molar mass 64.07 g/mol)
d. Cl2 (molar mass 70.90 5 g/mol)
e. N2O (molar mass 44.02 g/moll)
Answers
Ideal gas law is valid only for ideal gas not for vanderwaal gas. Therefore, correct option is option B that is CHCl[tex]_3[/tex]. Ideal gas is a hypothetical gas.
What is ideal gas equation?Ideal gas equation is the mathematical expression that relates pressure volume and temperature. There is no force of attraction between the particles.
Mathematically the relation between Pressure, volume and temperature can be given as
PV=nRT
where,
P = pressure of gas sample = 850 torr=1.11atm
V= volume of gas sample =0.918 L
n =number of moles of gas sample=?
T =temperature of gas =308K
R = Gas constant = 0.0821 L.atm/K.mol
Substituting all the given values, we get
1.11atm×0.918 L =n× 0.0821×308
On calculation we get
1.018=n×25.28
n=0.04moles
Molar mass =mass/moles
Molar mass = 4.84 g/0.04
=119. 4g/mol
Therefore, correct option is option B.
To learn more about ideal gas equation, here:
https://brainly.com/question/14826347
#SPJ1
determine the wavelength of the light emitted when the electron in a hydrogen atom undergoes a transition from n
Answers
The wavelength of light emitted when the electron in a hydrogen atom undergoes a transition from n is 486 nm.
What is wavelength?
Forms of electromagnetic radiation, such as radio waves, light waves, and infrared (thermal) waves, create unique patterns as they travel through space. Each wave has a specific shape and length. The distance between peaks (high points) is called the wavelength.
Therefore, The wavelength of light emitted when the electron in a hydrogen atom undergoes a transition from n is 486 nm. Forms of electromagnetic radiation, such as radio waves, light waves, and infrared (thermal) waves, create unique patterns as they travel through space. Each wave has a specific shape and length.
To learn more about wavelength refer the given link:-
https://brainly.com/question/9350992
#SPJ4
the text describes the first three reactions of a metabolic pathway. complete the sentences. not all the terms will be placed.a. FAD
b. Citrate
c. Isomerization
d. Alpha-ketagluterate
e. Isocitrate
f. Oxaloacetate
g. Succinate
h. NAD+
i. Intermediate
j. Product h. Condensationi. trycarboxylic acid1. In reaction 1 of the Krebs cycle, acetyl-CoA formed in the pyruvate dehydrogenase reaction condenses with the four-carbon compound____to form____with the elimination of coenzyme A. 2. Since the product has three carboxyl groups, this pathway is referred to as the____cycle. In reaction 2 of the Krebs cycle, this product then undergoes____to form_____. The enzyme is called aconitase because the compound cis-aconitate is the____of the reaction Reaction 3. eliminates Co, to form the five-carbon dicarboxylic acid____. Oxidation also occurs, with electrons transferred from the substrate to______. Consequently, this reaction is an oxidative decarboxylation.
Answers
In the first reaction of Krebs cycle (f)Oxaloacetate, (b)Citrate, (k)Tricarboxylic acid. in the second reaction (c)Isomerization, (e)isocitrate, (i)intermediate. and in the third reaction (d)alpha-ketoglutarate, (h)NAD+ .
In reaction 1 of the Krebs cycle, acetyl-CoA formed in the pyruvate dehydrogenase reaction condenses with the four-carbon compound Oxaloacetate to form citrate the elimination of coenzyme A. Since the product has three carboxyl groups, this pathway is referred to as tricarboxylic acid cycle. In reaction 2 of the Krebs cycle, this product then undergoes isomerization to form Isocitrate. The enzyme is called aconitase because the compound cis-aconitate is the intermediate of the reaction Reaction 3. eliminates Co, to form Alpha-ketoglutarate the five-carbon dicarboxylic acid. Oxidation also occurs, with electrons transferred from the substrate to NAD+. Consequently, this reaction is an oxidative decarboxylation.
To learn more about Krebs cycle please visit:
https://brainly.com/question/14738220
#SPJ4
In which type of chemical reaction do the positive ions switch between two compounds and form two brand new compounds?
Answers
In double displacement type of reaction the positive ions switch between two compounds and form two brand new compounds.
A double displacement is defined as a reaction in which the positive and negative ions of two ionic compounds exchange places to form two new compounds.
The double-displacement reaction generally represented in the form of AB + CD → AD + CB where A and C are positively-charged cations, while B and D are negatively-charged anions.
Some of the examples of double displacement reaction are given as,
NaOH + NH₄Cl ⇄ NaCl + NH₄OH
AgNO₃ + NaCl ⇄ AgCl + NaNO₃
Learn more about double displacement reaction from the link given below.
https://brainly.com/question/14195530
#SPJ4
What type of reaction is CH o2 co2 H2O?
Answers
The reaction CH₄ + O₂→ CO₂ + H₂O is a combustion reaction.
The balanced chemical equation of the reaction of Methane with Oxygen gas to produce carbon dioxide and water as a product is,
CH₄ + O₂→ CO₂ + H₂O
As you can see from the above reaction Methane is getting combusted because of the presence of the Oxygen gas. Methane is producing carbon dioxide as a product along with water.
So, we can conclude here that the above reaction is a combustion reaction. Also, it can be noted that it is also a kind of combination reaction because new products are forming because of the combination of two different reactants.
To know more about combustion reaction, visit,
https://brainly.com/question/13251946
#SPJ4
Select the correct answer. What is the noble gas electron configuration of bismuth (bi)? a. [kr] 6s2 6p3 b. [xe] 6s2 6p3 c. [xe] 4f14 5d10 6s2 6p3 d. [kr] 5s2 5d10 5p6 6s2 6d10 6p3 e. [xe] 6s2 5d10 6p3.
Answers
the noble gas electron configuration of bismuth (Bi) is [Xe] 4f¹⁴ 5d¹⁰ 6s² 6p³. The correct answer is C.
Bismuth is an element with atomic number 83 and contains 83 electrons. The next noble gas is sign xenon, which contains 54 electrons. Bismuth is an element belonging to group 15 of the periodic table.
Arsenic and bismuth are similar in that they are both metalloids and share more or less the same properties. This is predictable because they belong to the same group of the periodic table and elements in the same group are said to have more or less the same properties.
therefore, The electronic configuration of bismuth for the nearest noble gas is:[Xe] 4f¹⁴ 5d¹⁰ 6s² 6p³. The correct answer is C.
learn more about bismuth at https://brainly.com/question/28669107
#SPJ4
consider the following reaction at equilibrium. what effect will increasing the volume of the reaction mixture have on the system? 2 h2s(g) 3 o2(g) 2 h2o(g) 2 so2(g) a) the reaction will shift to the right in the direction of products. b) no effect will be observed. c) the reaction will shift to the left in the direction of reactants. d) the equilibrium constant will decrease. e) the equilibrium constant will increase.
Answers
Option (a) is correct. If a reaction is at equilibrium and we will increase the volume of the reaction then the Reaction will shift to the right in the direction of product.
The Reaction is,
2H2S(g) + 3O2(g) ----> 2H2O(g) + 2SO2(g) a)
According to Le Chatelier's principle, if a dynamic equilibrium is disturbed by changing the conditions the position of equilibrium shifts to counteract the change to reestablish an equilibrium. If a chemical reaction is at equilibrium and experiences a change in pressure, temperature or concentration of products or reactants, the equilibrium shifts in the opposite direction to offset the change. A change in one of the variables that describe a system at equilibrium produces a shift in the position of the equilibrium that counteracts the effect of this change.
We are increasing pressure here. Reaction will try to decrease the pressure. Hence it will move in a direction which have lesser gaseous molecules. Here product has less gaseous molecule. So equilibrium will move to right. Equilibrium moves to product side.
To learn more about Le chatelier's principle please visit:
https://brainly.com/question/2943338
#SPJ4
If you weigh out 0.205 g of salicylic acid, what is the theoretical yield for aspirin? 0.157g 0.267g 0.134g 0.0786g none of the above choices
Answers
If you weigh out 0.205 g of salicylic acid, the theoretical yield for aspirin is the 0.267 g.
given that :
the mass of the salicylic acid = 0.205 g
the molar mass of the salicylic acid = 138.12 g /mol
the number of moles of salicylic acid acid = mass / mola mass
= 0.205 / 138.12
= 0.001484 mol
1 mole of salicylic acid = 1 mole of the aspirin
0.001484 mol of the salicylic acid = 0.001484 mol of aspirin
the molas mass of aspirin = 180 g /mol
the mass of aspirin = moles × molar mass
= 0.001484 × 180
= 0.267 g
Thus the theoretical yield = 0.267 g.
To learn more about theoretical yield here
https://brainly.com/question/28203576
#SPJ4
the amino acids whose carbon skeleton are degraded in to precursors for the formation of glucose are called .
Answers
The amino acids whose carbon skeleton are degraded in to precursors for the formation of glucose are called is Glucogenic amino acids.
What is amino acids?
Proteins are made of substances called amino acids. The components of life are amino acids and proteins. Amino acids are what remain after proteins have been digested or broken down. In order to benefit the body: Food should be broken down.
What is glucose ?
The molecular formula of this monosaccharide is C6H12O6. It is also referred to as dextrose. Aldohexose is the name given to it since it has 6 carbon atoms and an aldehyde group. It comes in two varieties: open-chain and ring construction.
Amino acids that cause glucose production. The carbon skeletons are transformed into pyruvate, 2-oxoglutarate, succinyl-CoA, fumarate, and oxaloacetate, which serve as precursors to glucose.
Therefore, the amino acids whose carbon skeleton are degraded in to precursors for the formation of glucose are called is Glucogenic amino acids.
Learn more about amino acid from the given link.
https://brainly.com/question/14351754
#SPJ4
The movement of water and electrolytes between fluid compartments is regulated primarily by?
Answers
The movement of water and electrolytes between fluid compartments is regulated primarily by hydrostatic pressure and osmotic pressure.
Define electrolytes.
When dissolved in water, substances known as electrolytes acquire a natural positive or negative electrical charge.
The force that a fluid applies against a wall—hydrostatic pressure—is essentially moves fluid between compartments. The pressure that blood applies to the blood vessel walls as a result of the heart's pumping motion is known as the hydrostatic pressure of blood. The opposing "colloid osmotic pressure" in capillaries is greater than the blood's "constant" pressure, which is principally created by the circulation of albumin at the capillary's arteriolar end. This pressure pushes plasma and nutrients into the surrounding tissues from the capillaries.
Therefore, the movement of water and electrolytes between fluid compartments is regulated primarily by hydrostatic pressure and osmotic pressure.
To learn more about electrolytes from the given link.
https://brainly.com/question/17089766
#SPJ4
The inner planets are terrestrial and the outer planets are Jovian. Why?
Answers
The terrestrial planets originated near to the Sun, where the temperature was ideal for the condensing of rock and metal. Outside of the so-called frost line, where temperatures were low enough for ice condensation, the jovian planets formed.
Terrestrial Planets: Mercury, Venus, Earth, and Mars are examples of the terrestrial planets, which derive from the Latin word "terra," which means "land."
The remaining planets in the solar system (apart from Pluto)—Jupiter, Saturn, Neptune, and Uranus—are collectively referred to as the jovian planets. They were given this moniker because of their likeness to Jupiter.
Planets that are terrestrial have solid surfaces, a slower rotation, and less dense metal cores.
Jovian planets have non-solid surfaces, rapid rotation, and cores made of significantly denser hydrogen and metal.
For more information on planets kindly visit to
https://brainly.com/question/28459256
#SPJ4
a compound is found to have an empirical formula of ch2o. what is the molecular formula when the molecular weight is 180.0 grams?
Answers
The compound's empirical formula is CH2O, which contains two hydrogen atoms and one oxygen atom for every carbon atom.CH2O has a mass of 30 (=12+2*1+16).The molecule has a molecular weight of about 180.As a result, the supplied molecule has the molecular formula C6H12O6.
What is the chemical structure of the equation for water, CH2O?Therefore, a substance that is organic and has a molar mass pf 60 grams / mole as well as an empirical formula of CH2O also has a molecular formula or C2H4O2.
How much CH2O is in a mole?A mol of CH2O has a molar mass of 30.026 g.The periodic chart must first be used to estimate its atomic mass of the each element in the compound.
To know more about ch2o visit:
https://brainly.com/question/5523263
#SPJ4
A sample of gas weighs 4.84 g and occupies a volume of 0.918 l at 35 °c and 850 torr. identify the gas sample.
a. NH3 (molar = Mass 17,03 B/moll
b. CHCI3 (molar mass 119 4 B/mol)
c. SO2 (molar mass 64.07 g/mol)
d. Cl2 (molar mass 70.90 5 g/mol)
e. N2O (molar mass 44.02 g/moll)
Answers
The gas sample is CHCl₃ having molar mass of 119.135 g/mol.
Calculate the molar mass of the gas, by using the equation given by Ideal gas equation,
PV = nRT
PV = w/M RT
Where,
P = Pressure of the gas = 850 torr
V = Volume of the gas = 0.918 L
w = Weight of the gas = 4.84 g
M = Molar mass of gas = ?
R = Gas constant = 62.36 L
T = Temperature of the gas = [35 + 273] K = 308 K
Substituting the values we get,
850 torr × 0.918 L = 4.84 g/ M × 62.36 L × 308 K
⇒ M = (4.84 g× 62.36 L × 308 K) / (850 torr × 0.918 L)
⇒ M = 119.135 g/mol
Hence, the gas sample is CHCl₃ having molar mass of 119.135 g/mol.
Learn more about molar mass from the link given below.
https://brainly.com/question/14259285
#SPJ4
What is density of argon gas in g ml at STP?
Answers
1.78 g/L is density of argon gas in g ml at STP
To comparing various gas characteristics, standard temperature and pressure (STP) is a helpful set of reference conditions. Gases have a volume of 22.4 L per mole at STP. The ideal gas law may be used to calculate gas densities. The ideal gas law equation's variable "R" is referred to as the "gas constant." A constant number is always equal to the product of the pressure times the volume of a gas divided by the number of moles and the gas's temperature. The units that the temperature, pressure, and volume are expressed in determine the constant's numerical value.
Learn more about STP here:
brainly.com/question/15469566
#SPJ4
you found that you needed 27.50 ml of kmno4to titrate 10.0 ml 0f 0.095 m fe2 solution. what is the concentration of your potassium permanganate
Answers
The moles contained are 15 x 10⁻² M.
What is molarity ?
A chemical entity's concentration in some kind of a solution, specifically the quantity of a solute per unit volume of solution, has been measured by its molar concentration.
The equilibrium constant of a reaction determined by "The molarity of products is divided by the molarity of reactants".
moles of potassium dichromate = 0.095 x .027 = 25 x 10⁻⁵ moles
1 mole of potassium dichromate reacts with 6 moles of Fe⁺²
25 x 10⁻⁵ moles of potassium dichromate will react with
6 x 25 x 10⁻⁵ moles of Fe⁺²
= 1500 x 10⁻⁵ moles
150 x 10⁻⁵ moles are contained in 10 mL of solution
molarity of solution = 150 x 10⁻⁵ / 10 x 10⁻³
= 15 x 10⁻² M .
Therefore, moles contained are 15 x 10⁻² M
To know more about mole, refer: https://brainly.com/question/26416088
#SPJ4