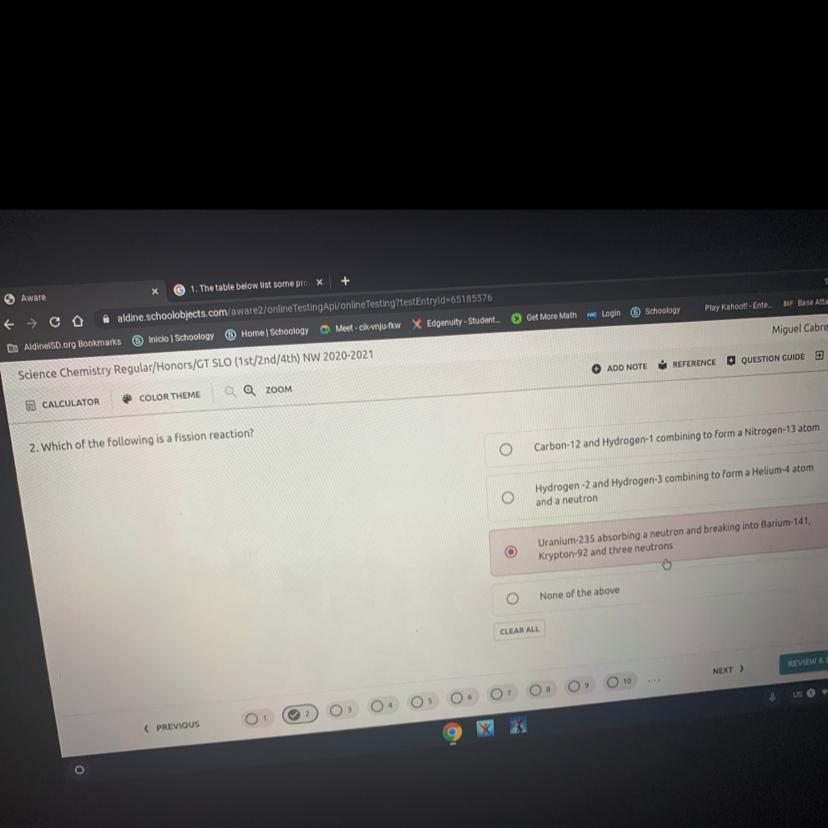
Please help me is my final 2. Which of the following is a fission reaction?
Carbon-12 and Hydrogen-1 combining to form a Nitrogen-13 atom
Hydrogen-2 and Hydrogen-3 combining to form a Helium-4 atom
and a neutron
Uranium-235 absorbing a neutron and breaking into Barium-141,
Krypton-92 and three neutrons
0
None of the above
0
Answers
Answer:
Uranium-235 absorbing a neutron and breaking into Barium-141, Krypton-92 and three neutrons
Explanation:
A fission reaction is said to have occurred when an unstable nucleus is bombarded with a neutron such that it disintegrates into daughter nuclei and produces more neutrons in the process. Hence, Uranium-235 absorbing a neutron and breaking into Barium-141, Krypton-92 and three neutrons is a fission reaction.
This fission reaction is often a self sustaining reaction. The neutrons produced in the reaction bombards more of the parent nucleus and the process continues indefinitely(chain reaction).
Control rods and moderators are used to keep the chain reaction in check.
Related Questions
10 points! Please help if you know the answer I need to get 100% on this test :)
Answers
Answer:
Metal has an high capacity, which allows it to heat up faster and transfer the heat to the contents of the pot or pan.
Explanation:
Because metal pots are made from a narrow range of metals because pots and pans need to conduct heat well.
During a combustion reaction, 9.00 grams of oxygen reacted with 3.00 grams of CH4.
What is the amount of the leftover reactant?
0.74 grams of methane
0.89 grams of methane
1.22 grams of oxygen
1.45 grams of oxygen
Answers
Answer:
0.74
Explanation:
this is the correct answer. I followed a correct answer that had the same probelm with different values, swithxed it with mine, and got 0.75. So its basically 0.74
Give three examples of energy traveling in waves
Answers
Answer:
ELECTROMAGNETIC SPECTRUM. Radios, televisions, mobile phones, and radar use signals made up of electromagnetic waves. These are waves that carry energy as electricity and magnetism at the speed of light. Light we can see is also an electromagnetic wave, but other types of electromagnetic wave are invisible.
Explanation:
~Hope this helps
Type the correct answer in the box. Express your answer to three significant figures. Iron(II) chloride and sodium carbonate react to make iron(II) carbonate and sodium chloride: FeCl2(aq) + Na2CO3(s) → FeCO3(s) + 2NaCl(aq). Given 1.24 liters of a 2.00 M solution of iron(II) chloride and unlimited sodium carbonate, how many grams of iron(II) carbonate can the reaction produce? The reaction can produce grams of iron(II) carbonate.
Answers
Answer:
Explanation:
To solve this problem we have to find the moles of iron(II) chloride that react. Using the chemical equation, we can kknow moles FeCl2 = Moles FeCO3. Thus, we can find the moles of FeCO3. Converting these moles to grams using its molar mass -Molar mass FeCO3: 115.854g/mol-
Moles FeCl2 = Moles FeCO3:
1.24L * (2.00mol / L) = 2.48 moles FeCl2
Mass FeCO3:
2.48mol * (115.854g / mol) =
The reaction can produce 287 grams of iron(II) carbonateAnswer:
its 287
Explanation:
i got it right on edm
PLEASE HELP QUICKLY!!
I know it's not A or B; I just can't figure it out between C and D.
---
What will most likely happen when a component is added to a reaction system at equilibrium?
A. The system will be unaffected by stress.
B. The addition of a catalyst will change the equilibrium.
C. The equilibrium position will shift away from what was added to lower its concentration.
D. The equilibrium position will shift away from what was added to increase its concentration.
Answers
Answer:
c: The equilibrium position will shift away from what was added to lower its concentration.
Explanation:
Answer: C. The equilibrium position will shift away from what was added to lower its concentration.
Explanation:
If we have a system that is already in equilibrium, the addition of an extra amount of one of the reactants or one of the products throws the system out of equilibrium. Either the forward or the reverse reaction will then occur to restore equilibrium conditions.
What type of reaction occurs when one element or ion within a compound is exchanged with another element or ion?
combustion
decomposition
single-displacement
double-displacement
Answers
Answer:
When an element replaces another in aone element or ion within a compound is exchanged with another element or ion , the reaction is called
single-displacementWhat element is this? Is it a neutral atom or ion?
Answers
Mr. Ragusa is approached in his lab by one of his students named Hassan. Hassan had found a bottle of nitric acid that did not have a labeled molarity and wanted Mr. Ragusa to help him find it's molarity. Mr. Ragusa filled a buret to 4.85 mL with 0.30 M NaOH. Mr. Ragusa added 5 mL of the acid to a flask and added some phenolphthalein. Mr. Ragusa hit the titration spot on, and the final volume in the buret was 43.69 mL. What is the concentration of the Nitric Acid?
Round your answer to 2 decimal places. Include a unit in your answer. (ex: 1.2M)
HNO3 + NaOH --> NaNO3 + H2O
Answers
Answer:
2.33M
Explanation:
1. First, make sure the equation is balanced. In this case it would be HNO3+NaOH --> NaNO3 + H2O. (it is already balanced here.)
2. Find the initial volume, final volume, the volume of acid, volume of NaOH.
a. Initial Volume: 4.85 mL
b. Final Volume: 43.69
c. Acid (HNO3) Volume: 5 mL
d Volume NaOH: Initial V - Final V --> 43.69 mL - 4.85 mL = 38.84 mL NaOH
3. Find moles of NaOH reacted using the volume from D. Convert this volume into Liters. Multiply the volume of NaOH by molarity of NaOH given to you before. (.30 M NaOH)
a. x mol NaOH/ .03884 L = .30 mol NaOH/ 1 mol = .011652 mol NaOH
4. Now find the moles of acid. The acid given to you in the equation is HNO3. The .011652 mol NaOH is from the moles reacted that you just found. The moles on the right side would be the co-efficient in front of the elements in the equation. Since this equation does not have any coefficients (it's already balanced), it would just be 1.
a. .011652 mol NaOH/ x mol HNO3 = 1 mol NaOH/ 1 mol HNO3.
x = .011652 mol HNO3
5. Now find the concentration, also known as molarity. Divide the .011652 mol by the volume of acid you have been given (5 mL). Before using 5 mL, divide it by 1000 to get the Liter form.
a. .011652 mol HNO3/ .005 L = 2.3304 M HNO3
I hope you understand now. God bless!
1) What experimental condition is required for the heat change in a reaction to be numerically equal to the enthalpy change (ΔH)?
2) When 1 mol of nitrogen gas reacts with 3 mol of hydrogen gas, 2 mol of ammonia gas is produced and 92.6 kJ of heat is released. Write the thermochemical equation.
Answers
Answer:
Answers are in the explanation
Explanation:
1) The constant pressure is necessary during a reaction. Thus, the heat released in the reaction will be = ΔH
2) The reaction described in the problem is:
1 N₂(g) + 3H₂(g) → 2NH₃(g)
The thermochemical equation involves the change in heat, as 92.6kJ are released, the heat must be wrote as another reactant, that is:
N₂(g) + 3H₂(g) → 2NH₃(g) + 92.6kJ71.1 mL of 0.695M rubidium hydroxide neutralized 89.7mL of sulfuric acid and solution of unknown concentration. Find the concentration of the acid
Answers
Answer: Concentration of the acid is 0.551 M.
Explanation:
Given: [tex]V_{1}[/tex] = 71.1 mL, [tex]M_{1}[/tex] = 0.695 M
[tex]V_{2}[/tex] = 89.7 mL, [tex]M_{2}[/tex] = ?
Formula used to calculate the concentration of acid is as follows.
[tex]M_{1}V_{1} = M_{2}V_{2}[/tex]
Substitute the values into above formula as follows.
[tex]M_{1}V_{1} = M_{2}V_{2}\\0.695 \times 71.1 mL = M_{2} \times 89.7 mL\\M_{2} = 0.551 M[/tex]
Thus, we can conclude that concentration of the acid is 0.551 M.
What is the pressure of 13.7 mol of acetylene in a 48.1 L cylinder at 67.0 °C?
Answers
Answer:
hinojosa has caught you cheating
Explanation:
ur mom
\
Solve for T in the ideal Gas Law!!!!!!
Answers
Answer:
T=PV/nR
Explanation:
PV=nRT then you just move them
is a shark asexual, sexual, or both
Answers
Answer:
Both
Explanation:
Please mark brailiest
Answer:
A shark is both
Explanation:
7. When a log is thrown on a fire it seems to lose weight; what happens to its physical mass?
a. It disappears
b. It is released into the air
C.
It becomes energy
d. The log does not change weight or size
Answers
Answer:I think it would be D.
Explanation:The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. For example, when wood burns, the mass of the soot, ashes, and gases equals the original mass of the charcoal and the oxygen when it first reacted. So the mass of the product equals the mass of the reactant.
what is the symbol of gold
Answers
Explanation:
Tha Symbol of gold is Au.
Answer:
symbol of gold is Au
------------------------------------------------
Give reasons why an element can be broken down...pls explain widely
Answers
If I initially have a gas at a pressure of 1260 kPa, a volume of 23 liters, and a temperature of 200 K, and
then I raise the pressure to 1595 kPa and increase the temperature to 300 K, what is the new volume of
the gas?
Answers
______ = ________
T1 T2
1260 x 23 / 200 = 144.9
1595x V/300
144.9= 1595x V/300
144.9 x 300/1595 = v
27.25 litres
I think
What will the change in temperature be when 90 J are applied to 15 g of gold. (cgold = 0.126 J/g°C)
Answers
Answer:
47.6°C is the change of temperature
Explanation:
To solve this question we must use the equation of specific heat of a material:
Q = m*ΔT*S
Where Q is heat applied = 90J
m is the mass of the substance = 15g gold
ΔT is change in temperature = Our incognite
S is specific heat of the material = 0.126J/g°C for gold
Replacing:
90J = 15g*ΔT*0.126J/g°C
90J/15g*0.126J/g°C = ΔT
ΔT = 47.6°C is the change of temperature
A student carried out a titration using HC2H3O2(aq)HC2H3O2(aq) and NaOH(aq)NaOH(aq). The net ionic equation for the neutralization reaction that occurs during the titration is represented above. The NaOH(aq)NaOH(aq) was added from a buret to the HC2H3O2(aq)HC2H3O2(aq) in a flask. The equivalence point was reached when a total of 20.0mL20.0mL of NaOH(aq)NaOH(aq) had been added to the flask. How does the amount of HC2H3O2(aq)HC2H3O2(aq) in the flask after the addition of 5.0mL5.0mL of NaOH(aq)NaOH(aq) compare to the amount of HC2H3O2(aq)HC2H3O2(aq) in the flask after the addition of 1.0mL1.0mL of NaOH(aq)NaOH(aq), and what is the reason for this result
Answers
Answer:
The amount of HC₂H3₃2(aq) in the flask after the addition of 5.0mL of NaOH(aq) compared to the amount of HC₂H₃O₂(aq) in the flask after the addition of 1.0mL is much smaller because more HC₂H₃O₂(aq) is required to react with 5.0 mL NaOH than with 1.0 mL NaOH.
Explanation:
Equation of the reaction between acetic acid, HC₂H₃O₂(aq) and sodium hydroxide, NaOH(aq) is given below:
CH₃COOH (aq) + NaOH (aq) ----> CH₃COONa (aq) + H₂O
The equation of the reaction shows that acetic acid andsodium hydroxide will react in a 1:1 ratio
Since the concentration of NaOH was not given, we can assume that the concentration is 0.01 M
Moles of NaOH in 5.0 mL of 0.01 M NaOH = 0.01 × 5/1000 = 0.00005 moles
Moles of NaOH in 1.0 mL of 0.01 M NaOH = 0.01 ×1/1000 = 0.0001 moles
Ratio of moles of NaOH in 5.0 mL to 1.0 mL = 0.00005/0.00001 = 5
There are five times more moles of NaOH in 5.0 mL than in 1.0 mL and this means that 5 times more the quantity of HC₂H₃O2(aq) required to react with 1.0 mL NaoH is needed to react with 5.0 mL NaOH.
Therefore, the amount of HC₂H₃O2(aq) in the flask after the addition of 5.0mL of NaOH(aq) compared to the amount of HC₂H₃O₂(aq) in the flask after the addition of 1.0mL is much smaller because more HC₂H₃O₂(aq) is required to react with 5.0 mL NaOH than with 1.0 mL NaOH.
Lead, gold mercury, aluminum which one has the lowest heat capacity?
Answers
Which statement is true in comparison to a weak acid, a strong acid
has a larger ph than weak acids
is more concentrated than we gases
strong acids produced less H plus ions is mostly H plus ions in solution
Answers
Answer: Strong acid vs weak acid
Strong acids and strong bases refer to species that completely dissociate to form ions in solution.
Explanation: By contrast, weak acids and bases ionize only partially, and the ionization reaction is reversible. Thus, weak acid and base solutions contain multiple charged and uncharged species in dynamic equilibrium.
Need this asap!!!
Which type of substance gives off hydroxide ions when dissolved in water?
Acid
Base
Gas
Metal
Answers
Answer:
Acid
Explanation:
On a hot day, a 15.0 kg window increased from 20.0 degrees C to 26.7 degrees C. How much heat energy did the glass window absorb? (specific heat of glass = 0.840 J/gC)
Answers
Answer: The heat energy absorbed by the glass window is 84420 J.
Explanation:
Given: Mass = 15.0 kg (1 kg = 1000 g) = 15000 g
Specific heat capacity = [tex]0.840^{o}C[/tex]
Initial temperature = [tex]20^{o}C[/tex]
Final temperature = [tex]26.7^{o}C[/tex]
Formula used to calculate heat energy is as follows.
[tex]q = m \times C \times (T_{2} - T_{1})[/tex]
where,
q = heat energy
m = mass of substance
C = specific heat capacity
[tex]T_{1}[/tex] = initial temperature
[tex]T_{2}[/tex] = final temperature
Substitute the values into above formula as follows.
[tex]q = m \times C \times (T_{2} - T_{1})\\= 15000 g \times 0.840J/g^{o}C \times (26.7 - 20.0)^{o}C\\= 84420 J[/tex]
Thus, we can conclude that heat energy absorbed by the glass window is 84420J.
Which species is the reducing agent in a redox reaction? Chem is hard
Answers
Answer:
The species that furnishes the electrons is called the reducing agent. In this case, the reducing agent is zinc metal.Explanation:
Hopes this helps. Mark as brainlest plz!In a redox reaction, the species which is oxidized acts as the reducing agent.
In a redox reaction, The substance losing electrons is the reducing agent and is being oxidised. The substance that gains electrons is the oxidising agent and is being reduced.
An oxidising agent isdefined as a substance that causes oxidation by accepting electrons and thus becoming reduced.
A reducing agent is defined as a substance that reduces by losing electrons and thus becomes oxidised.
Substances that have the ability to oxidise other substances are referred to as oxidising agents or oxidants
Substances that have the ability to reduce other substances are referred to as reducing agents or reductants.
To know more about Redox reaction
brainly.com/question/13293425
#SPJ2
The question should be
Which species is the reducing agent in a redox reaction?
Someone help me with this question please
Answers
Answer:
step 2
Explanation:
ofcourse rocks dont melt
maybe lol
What are the three parts of modern cell theory. Choose all that apply.
A.) The cell is the smallest living unit in organisms
B.) All cells come from other pre-existing cells
C.) All living things are made of cells
D.) All organisms are multicellular
Answers
Answer:
a,b,c
Explanation:
Answer:
C
...................
Which is a physical change? A) Rusting iron B) Burning paper C) Reacting baking soda and vinegar D) Separating a mixture of salt and wat
Answers
Answer:
D
Explanation:
Select the correct structure that
corresponds to the name.
4-bromo-5-chlorocyclohexene
Answers
Answer: a
Explanation:
Balance the following equations.
Answers
Answer:
A) Mg + H2SO4 → MgSO4 + H2
B) CaCO3 + 2HCl → CaCl2 + H2O + CO2
C) CaO + H2O → Ca(OH)2
D) 3Ag + 4HNO3 → 3AgNO3 + H2O + NO2
Explanation:
A) Mg + H2SO4 → MgSO4 + H2
The equation is already balanced because the number of each element in the left hand side is already equal to that in the right hand side.
B) CaCO3 + 2HCl → CaCl2 + H2O + CO2
This equation is balanced by putting 2 in front of HCl on the left hand side to balance the elements on the right hand side.
C) CaO + H2O → Ca(OH)2
Thus equation is already balanced because the number of each element in the left hand side is already equal to that in the right hand side.
D) 3Ag + 4HNO3 → 3AgNO3 + H2O + NO2
This equation is balanced by putting 3 in front of Ag and 4 in front of HNO3 on the left hand side while we put 3 in front of AgNO3 on the right side to balance the elements on the right hand side.
Which of the following has the most energy?
heat
ultraviolet rays
the color blue
radio waves
Answers
Answer:
C
Explanation: