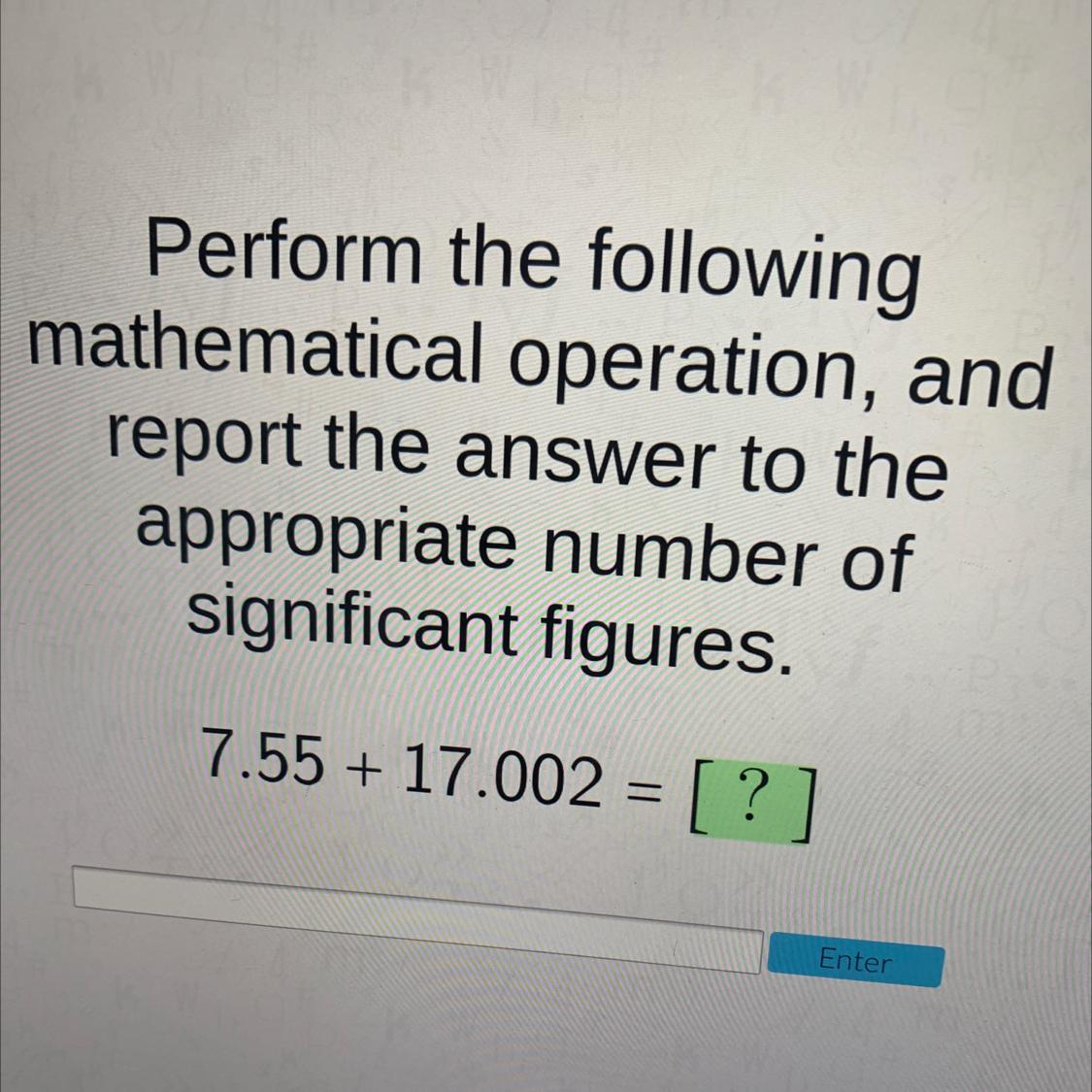
Answers
7.55 + 17.002 = ?
First, we have to determine the number of significant figures that each number has.
7.55: 3 SF and 2 SF after the decimal point.
17.002: 5 SF and 3 SF after the decial point.
Second, perform the mathematical operation as we usually do.
7.55 + 17.002 = 24.552
Finally we determine the correct number of SF. When adding or substracting the rule says: "The final answer may have no more SF after the decimal point than the least number of significant figures in the operation". Since 7.55 has 2 SF after the decimal point, we must round the result of the addition to the second place to the right of the decimal point.
Rounding 24.552 we get 24.55.
Answer: 24.55
Related Questions
Which part of an amino acid is different between the 20 different amino acids?• R-Group• Nitrogen Group• H-Group• COOH Group
Answers
The sides groups are what make each amino acid different from the others. Of the 20 side groups used to make proteins, there are two main groups: polar and non-polar. These names refer to the way the side groups, sometimes called "R" groups.
Using that,
In conclusion, the part of an amino acid that is different between the 20 different amino acids is the R-Group.
ANSWER:
• R-Group
I need help with my homework I need help with number 3Given: 1 foot is equivalent to 12 inches Given: 1 yard is equivalent to 3 feet Given: 1 mile is equivalent to 5, 280 feet The number of yards in 0.45 miles is
Answers
Answer:
The number of yards is 792.
Explanation:
To convert 0.45 miles to yards it is necessary to use the equivalence from mile to feet, and then the equivalence from feet to yard:
[tex]0.45\text{miles}\cdot(\frac{5,280feet}{1\text{mile}})\cdot(\frac{1\text{yard}}{3\text{feet}})=792\text{yards}[/tex]So, it is 792 yards.
To reduce dangerous swelling in the brain, physician sometimes administer intravenous hypertonic saline, a 5.0%(w/v) Solution of salt (NaCI) In water. Calculate the volume of the solution that contains 0.50 g of NaCl. Be sure your answer has unit symbol and his rounded to the correct number of significant digits
Answers
Percent weight/volume (% w/v) is a way of expressing the concentration of a solutuon. The definition of it is:
% w/v = mass of solute /100 ml of solution
We have to find the volume of the 5.0 % (w/v) solution that contains 0.50 g of NaCl. So we are given these values:
Concentration % w/v = 5.0 %
mass of solute = 0.50 g
Since our solution is 5.0 % w/v Nacl, we know that it has 5.0 g NaCl in 100 ml of solution. So if we want to find the volume of solution that contains 0.50 g we can:
0.50 g of NaCl * 100 ml of solution/(5.0 g of NaCl) = 10. ml of solution.
10. mL of solution is the volume that contains 0.50 g of NaCl
29.Which of the following is not an example of an acid-base indicator?Select one:a. Phenolphthaleinb. Litmus paperc. Hydrion paperd. Filter paper
Answers
Answer:
[tex]D\text{ : Filter Paper}[/tex]Explanation:
Here, we want to select the option that is not an acid-base indicator from the options
Acid-base indicators are colored materials that are expected to change color based on the pH
A filter paper is a material used in filtration
As such, it cannot be used as an indicator
Calculate the % composition of Al2O3?Group of answer choices47.1% AL, 52.9% O52.9% Al, 47.1% O28.3% Al, 71.7% O71.1% Al, 28>3% O62.8% Al, 37.2% O
Answers
First, we need to find the values of molar mass of Al and O at the periodic table:
Al : 27 g/mol
O: 16 g/mol
Now let's calculate the molar mass of Al2O3:
(2x27) + (16x3)
54 + 48 = 102 g/mol
Now let's calculate the percent of each element:
For Al:
102 ---- 100%
54 ---- x%
x = 52.9%
For O:
102 ---- 100%
48 ---- y%
y = 47.1%
Answer: 52.9% Al, 47.1% O
Determine the mole fraction of methanol CH3OH and water is a solution prepared by dissolving 4.8 g of alcohol in 38 g of H2O.
Answers
Answer
Mole fraction of water = 0.066
Explanation
Given:
mass of methanol = 4.8 g
mass of water = 38 g
We know:
Molar mass of methanol = 32,04 g/mol
Molar mass of water = 18,01528 g/mol
Solution:
Step 1: Find the moles of methanol and water
n of methanol = m/M
n = 4.8g/32.04g/mol
n = 0.15 mol
n of water = 38g/18.01528
n = 2.11 mol
now mole fraction of methanol= mole of methanol/(mole of methanol + mole of water)
Mole fraction of methanol = 0.15mol/(0.15 mol + 2.11 mol)
Mole fraction of water = 0.066
A jug of vinegar is 15% (by mass) acetic acid. If a container holds 500 g of this product, how much acetic acid does it contain?
Answers
Answer:
It contains 75g of acetic acid.
Explanation:
With the percent mass (15%) and the mass of the solution of vinegar, we can calculate the amount of acetic acid, using the percent by mass formula:
[tex]\begin{gathered} PercentByMass=\frac{MassOfSolute}{MassOfSolution}*100\% \\ 15\%=\frac{MassOfSolute}{500g}\times100\operatorname{\%} \\ \frac{15\%}{100\operatorname{\%}}*500g=MassOfSolute \\ 75g=MassOfSolute \\ \end{gathered}[/tex]So, it contains 75g of acetic acid.
Maria is an 18 year old girl whose parathyroid has stopped functioning properly. What does this mean for the calcium levels in her
body?
Answers
Maria is an 18 year old girl whose parathyroid has stopped functioning properly, this mean calcium levels in her body decreased.
Calcium (Ca) is a chemical element and an alkaline-earth metal from Periodic Table Group 2 (IIa). It is the fifth most abundant element in the Earth's crust and the most prevalent metallic element in human tissue.
The ancients made considerable use of lime (calcium oxide, CaO), a calcium compound. Sir Humphry Davy first extracted the lightweight, silvery metal itself in 1808 after distilling mercury from an amalgam created by electrolyzing a combination of lime and mercuric oxide. The Latin word for lime, calx, served as the inspiration for the element's name. The cosmic abundance of calcium is thought to be 4.9 104 atoms, and it makes up 3.64 percent of the crust of the Earth and 8 percent of the crust of the Moon.
To know more about calcium visit : https://brainly.com/question/3569638
#SPJ9
You dissolve 123g KBr into 689g of water. Calculate the mass percent.
Answers
Step 1
% by mass is defined as:
mass of solute ------ 100 g of solution
------------------
Step 2
The mass of the solution = mass of solute (KBr) + mass of solvent (water) = 123 g + 689 g = 812 g
------------------
Step 3
Procedure:
123 g KBr ------ 812 g solution
X ------ 100 g solution
X = 100 g solution x 123 g KBr/812 g solution = 15.1 g KBr = 15.1 % by mass
Answer: 15.1 % by mass
How do electrolytes conduct electricity
Answers
The electrolyte can conduct electricity because the molecules are broken up into ions.
When dissolved in water, electrolytes are compounds that release ions. Salts, bases, and acids can all be classified among them. Due to the mobility of the positive and negative ions, known as cations and anions, respectively, these solutions transmit electricity. For instance, the electrolyte of a lead-acid automobile battery is diluted sulfuric acid, which includes both positive hydrogen ions (H+, a cation) and negative sulphate ions (SO42-, an anion).
Electrolytes can be ionic compounds that dissociate into cations and anions when dissolved (such as ionic salts) or covalent chemicals that chemically react with water to form ions (such as acids and bases). When dissolved in water, compounds known as nonelectrolytes do not create ions.
To know more about electrolyte visit : https://brainly.com/question/28699046
#SPJ9
Which of the following would likely be a non -conductor of electricity?a) An aqueous solution of magnesium sulfate (Epsom salts)b) Gasolinec) An aqueous solute of sodium carbonate (washing powder)d) Carbonated soft drinksExplain your answer.
Answers
This would be the Gasoline, letter B, since gasoline is a hydrocarbon and these compounds are formed with covalent bonds, they share electrons and not gain or lose, forming ions and then conducting electricity, as we can see in the other 3 options, they all form ions.
Q: Use the equation to calculate the following:2 NaOH(aq) + H1S04(aq)- Na2SO4(aq) + 2 H2O(/)(a) the moles of Na2SO4 produced from 3.6 mol H2SO4?
Answers
1) First, let's write the equation:
NaOH(aq)
Please Help me solve ksp of AgOH = 2.0 x 10^-8
Answers
Explanation:
Silver hydroxide is almost an insoluble compound, its solubility product is 2*10^(-8). When it is in solution it dissolves like this way.
AgOH ----> Ag⁺ + OH⁻ Ksp = 2 * 10^(-8)
The expression for the solubilty product will be:
Ksp = [Ag+] * [OH-]
We are trying to dissolve the silver hydroxide in the silver nitrate solution. They have one ion in common, the silver cation (Ag+). Since the amount of silver hydroxide that can be dissolved is really small (because it is almost insoluble) we can consider that the concentration of the silver ion is governed by the concentration of the silver nitrate solution.
AgNO₃ ----> Ag+ + NO₃-
Since one molecule of silver nitrate has one nitrate ion we can say that the concentration of the silver ion will be the same as the concentration of the silver nitrate solution.
[AgNO₃] = [Ag+] = 0.270 M
Now that we know the concentration of the silver ion and the ksp, we can replace these values and find the concentration of the hydroxide ion.
Ksp = [Ag+] * [OH-]
[OH-] = Ksp/[Ag+]
[OH-] = 2 * 10^(-8)/(0.270 M)
[OH-] = 7.41 *10^(-8) M = [AgOH]
Answer: the maximum amount of silver hydroxide that will dissolve it 7.41*10^(-8) M
What is the following of element P and P^3- :Orbital DiagramLong NotationShort NotationValence LevelGroup on Periodic TablePeriod on Periodic Table
Answers
We are asked to find some characteristics of phosphorus. We start with the yu period group to which they belong in the periodic table. This information is found in the periodic table of elements.
Looking in the periodic table we find that phosphorus P is located in group VA (15) and in period 3.
The electronic configuration depends on the number of electrons of the element. Phosphorous has 15 electrons. We have an ion P^3-, this ion has three more electrons, so for this element, we will have 18 electrons. The electronic configuration will be:
The short notation depends of another element, in this case of Ne, so the shot notation will be:
[tex]\lbrack Ne\rbrack3s^23p^6[/tex]The valence level is the last energy level, we see that the greatest coefficient is 3, so the valence level is 3
In summary, we have that the answer will be:
Orbital Diagram: Drawn in the picture
Long Notation: 1s2 2s2 2p6 3s2 3p6
Short Notation: [Ne] 3s2 3p6
Valence Level: 3
Group on Periodic Table: 15 or VA
Period on Periodic Table: 3
The most prominent line in the spectrum of magnesium is 285.2 nm. Other lines are found at 383.8 and 518.4 nm.
What is the energy of 1 photon of the light with the wavelength of the most energetic line?
Answers
The energy of the 1 photon of the light with the wavelength of the most energetic line is 6.9 * 10^-19 J.
What is the energy of a photon?We know that a photon can only be explained as simply as possible as a bundle of light. The photon is emitted from an atom when an electron in the atom is seen to move from a higher to a lower energy level thereby emitting light of the appropriate wavelength.
Now we can see that the wavelength that is the shortest is the one that would have the greatest energy. This is because the energy of the photon is inversely proportional to the energy of the photon.
Now we have';
Wavelength of the photon = 285.2 nm or 285.2 * 10^-9 m
Speed of light = 3 * 10^8 m/s
Plank's constant = 6.6 * 10^-34 J/s
Then;
E = 6.6 * 10^-34 J/s * 3 * 10^8 m/s/ 285.2 * 10^-9 m
E = 6.9 * 10^-19 J
Learn more about wavelength:https://brainly.com/question/13533093
#SPJ1
Write All Gas Laws Meaning And Example Situation.
Answers
Gas laws discover the relationship of pressure, temperature, volume and amount of gas.
1. Boyles Law
Boyle's Law tells us that the volume of gas increases as the pressure decreases, meaning pressure is inversely propotional to volume.
Equation used for Boyles Law: P1V1 = P2V2
Example: A 17.50mL sample of gas is at 4.500 atm. What will be the volume if the pressure becomes 1.500 atm, with a fixed amount of gas and temperature?
So in this example we are given, P1 and P2, we are also given V1 and we want to know V2. What will happen to the volume if the pressure is increased.
V2 = P1V1/P2
V2 = (4.500 atm x 17.50 mL)/1.500atm
V2 = 52.50mL
This example proves that when the pressure is increased, the volume also increases.
2. Charles Law
Charles' Law tells us that the volume of gas increases as the temperature increases. Volume is directly proportional to Temperature.
The equation used: V1/T1 = V2/T2
Example: A sample of Carbon dioxide in a pump has volume of 20.5 mL and it is at 40.0 oC (=313.15 K). When the amount of gas and pressure remain constant, find the new volume of Carbon dioxide in the pump if temperature is increased to 65.0 oC (=333.15 K).
V2 = (V1 x T2)/T1
V2 = (20.5 mL x 333.15 K)/313.15K
V2 = 21.8 mL
The volume increased as the temperature increased.
3. Avogadro's Law tell us that the volume of gas increases as the amount of gas increases. Volume(V) is directly proportional to the Amount of gas(n).
Equation: P1/n1 = P2/n2 and V1/n1 = V2/n2
Example: A 3.80 g of oxygen gas in a pump has volume of 150 mL. constant temperature and pressure. If 1.20g of oxygen gas is added into the pump. What will be the new volume of oxygen gas in the pump if temperature and pressure held constant?
This problem is asking for V2 of oxygen. We have to first calculate the number of moles of Oxygen.
n1 = 3.80/(15.999 x 2)
n1 = 0.1188 mol
n2 = 1.20/(15.999 X 2)
n2 = 0.0375 mol
V2 = (V1 x n2)/n1
V2 = (150.0mL x 0.0375)/0.1188
V2 = 47.3485 mL
4. The ideal gas law
The ideal gas law is the combination of the three simple gas laws.
Equation: pV = nRT
Example: At 655mm Hg and 25.0oC, a sample of Chlorine gas has volume of 750mL. How many moles of Chlorine gas at this condition?
So we want number of moles.
n = PV/RT
n = 655mmHg x( 1 atm/760 mmHg) x 0.75 L/0.082057L⋅atm⋅mol-1.K-1 x 298.15
n = 0.026 mol
Select the structure that correspondsto the name.methyl butanoateA.iB.CH3OCCH₂CH₂CH3 CH3CH₂COCH 3C. both
Answers
The structure that corresponds to methyl butanoate is CH₃CH₂CH₂CO₂CH₃.
The chemical formula for methyl butanoate is C₅H₁₀O₂. It is also called methyl butyrate. Since the chemical formula is methyl butyrate, the structure should contain a chain of 4 Carbon atoms, one methyl group, and a keto group, that is C=O structure in it.
Methyl butanoate is an organic compound in the group of fatty ester acids. These compounds contains a fatty acid that is esterified with a methyl group.The general structure of the formula is RC(=O)OR' where R is the fatty aliphatic tail or organyl group and R' is the methyl group.
That is why the structure contains two oxygen atoms and a methyl group in it. Thus the structure of methyl butanoate is given by, CH₃CH₂CH₂CO₂CH₃.
To know more about organic compounds, click below:
https://brainly.com/question/1594044
#SPJ9
can someone help me with this question
Answers
Explanation:
DataP = 1.3 atm
V = 2.1 L
T = 75 °C = 75 + 273 = 348 K
R = 0.0821 atm.L / mol.K
n = ?
SolutionAs, PV = nRT
therefore, n = PV/RT
n = 1.3 x 2.1 / 0.0821 x 348
n = 0.096 mole
What is the percent concentration of a drug if 255 mg of the drug is found in 650 mL? Round your final answer to 3 decimal places if necessary.
Answers
1) List the known values.
Solute: 255 mg
Solution: 650 mL
Unknown
Percent concentration:
2) Set the equation
[tex]\frac{mass}{volume}\%=\frac{grams\text{ }of\text{ }solute}{milliliters\text{ }of\text{ }solution}*100[/tex]Convert milligrams to grams
1 g = 1000 mg
[tex]g=255mg*\frac{1g}{1000mg}=0.255g[/tex]3) Plug in known values.
[tex]\frac{mass}{volume}\%=\frac{0.255g\text{ }of\text{ }solute}{650\text{ }mL\text{ }of\text{ }solution}*100[/tex][tex]=0.039231\%[/tex]The percent concentration of the drug is 0.039%(m/V)
.
How are the chlorate and chlorite ions different from each other?How are they similar to the sulfate and sulfite ions?
Answers
Explanation and Answer:
formula of Chlorite ion = ClO₂⁻
formula of Chlorate ion = ClO₃⁻
The chlorite ion contains two atoms of O, and the chlorate ion contains three atoms of O.
They are both cations and their charges are -1 but, the main difference between them is that the oxidation state of Cl in the chlorite ion is 3+ and the oxidation state of Cl in the chlorate ion is +5.
formula of sulfite ion = SO₃²⁻
formula of sulfate ion = SO₄²⁻
Sulfite and sulfate are both cations with charge -2. The oxidation state of S in the sulfite ion is +4 and the oxidation state of S in the sulfate ion is +6.
How are the chlorate and the chlorite ions similar to the sulfate and sulfite ions?
When naming a polyatomic ion the sufix is related to the oxidation state of the main element.
common positive oxidation states of Cl = +1 +3 +5 +7
common positive oxidation states of S = +4 +6
When the element has two main positive oxidation states (like S) , the sufix for the lower oxidation state is -ite (sulfite = +4) and the sufix for the greater oxidation that is usually more stable is -ate (sulfate = +6).
When the element has four main positive oxidation states (like Cl), the two in the middle are named in a similar way to the previous one. The lower oxidation state must have -ite as a sufix (chlorite = +3) and the sufix for the greater is -ate (chlorate = +5).
Ions are the charged chemical species. Chlorate and chloride are two different ions of chlorine which are represented as ClO₃⁻ and ClO₂⁻, respectively. These are similar to sulfate and sulfite ions in the difference in the charge present on them.
What are ions?
An ion is an atom or a molecule which carry a net electrical charge. The electric charge is a result of the valency of the atom.
Chlorite and chlorate are the two charged ions of chlorine and can be represented as:
Formula of Chlorite ion = ClO₂⁻
Formula of Chlorate ion = ClO₃⁻
The chlorite ion contains two atoms of O, whereas the chlorate ion contains three atoms of O. Both of these ions are anions as they carry a net negative charge with a charge of -1. The main difference between these two is the oxidation state of Cl in the chlorite ion which is +3 and the oxidation state of Cl in the chlorate ion is +5.
The sulfate and sulfite ions are the two charges species of sulfur which can be represented by:
formula of sulfite ion = SO₃²⁻
formula of sulfate ion = SO₄²⁻
Sulfite and sulfate are anions with a charge of -2. The oxidation state of sulfur in the sulfite ion is +4 and the oxidation state of sulfur in the sulfate ion is +6.
These are similar to chlorate and chloride ions in oxidation states which are: Oxidation state of Cl = +1, +3, +5, and +7 and Oxidation states of S = +4 and +6
Learn more about Ions here:
https://brainly.com/question/14982375
#SPJ2
A chemist wants to make 7,837 mL of a solution with a concentration of 2.45 M.How many liters of a 6.85 M solution should be used to make this solution?
Answers
AnswerExplanation:
The liters of a 6.85 M solution that should be used to make the solution can be calculated using the dilution formula.
The formula for calculating dilutions is:
[tex]C_1V_1=C_2V_2[/tex]C₁ = 2.45 M
V₁ = 7837 mL = 7837/1000 = 7.837 L
C₂ = 6.85 M
V₂ = unknown
The unknown volume, V₂ is the required volume needed to make the solution.
Therefore, the V₂ can be calculated using the formula above as follows:
[tex]undefined[/tex]You are preparing a solution from 750 L of a 3.0 M NaCl solution.
How many L of a 1.5 M NaCl solution could you produce by
diluting the original solution?
O 0.006 L
O 6L
Q 3000 L
Q 1500 L
Q 375 L
Answers
You are preparing a solution from 750 L of a 3.0 M NaCl solution then 1.5 M NaCl solution could you produce by diluting the original solution is 1500L
Solution is a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to what is called the limit of solubility.
Here given data is
Volume = V₁ = 750 L
M₁ = 3.0 M
M₂ = 1.5 M
V₂ = ?
We have to calculate L of a 1.5 M NaCl solution could you produce by diluting the original solution = ?
So the formula is
M₁V₁ = M₂V₂
3.0 M ×750 L = 1.5 M ×V₂
V₂ = 1500L
Know more about volume
https://brainly.com/question/3677716
#SPJ1
What volume of 1.00 M HCl in liters is needed to react completely (with nothing left over) with 0.500 L of 0.500 M Na2CO3 ?Express your answer to three significant figures and include the appropriate units.
Answers
ANSWER
The volume of the acid is 0.5 L
EXPLANATION
Given that;
The molarity of HCl is 1.00 M
The volume of Na2CO3 is 0.500 L
The molarity of Na2CO3 is 0.500M
To find the volume of HCl needed, follow the steps below
Step 1; Write the balanced equation for the reaction
[tex]\text{ 2HCl\lparen aq\rparen }+\text{ Na}_2CO_{3(aq)}\text{ }\rightarrow\text{ 2NaCl}_{(aq)}\text{ }+\text{ CO}_{2(g)}\text{ }+\text{ H}_2O_{(l)}[/tex]In the above reaction, 2 moles of HCl is needed to completely react with Na2CO3 .
Step 2; Apply the molarity concept to find the volume of the acid
[tex]\text{ }\frac{C_AV_A}{C_BV_B}\text{ = }\frac{n_A}{n_B}[/tex]Where
CA is the concentration of the acid
VA is the volume of acid
CB is the concentration of the base
VB is the volume of the base
nA is the number of moles of acid
nB is the number of moles of base
Step 3; Substitute the given data into the formula in step 2
[tex]\begin{gathered} \text{ }\frac{1\text{ }\times\text{ V}_A}{0.5\text{ }\times\text{ 0.5}}\text{ = }\frac{2}{1} \\ \text{ Cross multiply} \\ \text{ 1 }\times V_A\text{ = 2 }\times\text{ 0.5 }\times\text{ 0.5} \\ \text{ V}_A\text{ = 0.5 L} \end{gathered}[/tex]Hence, the volume of the acid is 0.5 L
Can you help me answer 13 and explain the answer
Answers
Assuming that this question is comparing two situations, only water as situation 1 and water + calcium chloride as situation 2. In this case, we will have the salt interacting with water in something called Colligative properties, which depending on the concentration of the solute, it will cause changes in physical state change of the solution. Salts in water tend to cause two things, regarding physical change, they raise the boiling point and lower the freezing point. Which is the case for this question, we have a solution with salt + water, therefore we will have a higher boiling point and a lower freezing point, number 3
What is the purpose in the procedure of chemistry lab: Titration?
Answers
1) Titration. This is a common laboratory method used to determine the unknown concentration of a substance.
2) We use a solution with a known concentration. This solution can be an acid (or a base). We fill a burette with this solution.
3) In a flask, we have the solution with unknown concentration and a known volume. It can be a base (or an acid).
4) Let the solution out of the burette and into the flask. We have to do this until the indicator changes color. Read the volume in the burette.
5) Finally, we have to calculate the concentration of the solution.
What is the chemical reaction for calcium
Answers
Answer:
I think the answer is calcium reacts with oxygen, forming a thin layer of CaO
Explanation:
I hope this helps
The reaction 2Al + 6HCl -> 2AlCl3 +3H2 is classified as a:
Answers
You can see that aluminum (in the reactant) has an oxidation state of zero. Remember that when an element is 'alone' in a reaction, its oxidation state is always zero. In the case of HCl, H has the oxidation number of +1 and Cl -1, the algebraic sum between these two elements is zero.
In the products, the oxidation state of chlorine (Cl) remains unchanged whereas aluminum (Al) has the oxidation state of +3. Doing the algebraic sum of these ox. states of AlCl3, we obtain: -1 (3) + 3 = -3 +3 = 0. And hydrogen (H2) has an oxidation state of zero.
You can realize that aluminum is oxidating because it's changing its ox. state from 0 to +3 and hydrogen is reducing from +1 to 0, meaning that this is an oxidation-reduction reaction.
There are such types of chemical reactions. For this redox reaction, we can identify easily that the type of reaction is a single replacement reaction. A single-replacement reaction is a reaction in which one element replaces a similar element in a compound. We're replacin
Calculate the amount of heat needed to boil 41.1 g of water (H2O), beginning from a temperature of 84.7 C . Be sure your answer has a unit symbol and the correct number of significant digits.
Answers
Explanation:
We need to go through to stages to boil 41.1 g of water. We have to heat the sample of water from 84.7 °C to 100 °C (the boiling point) And then we have to provide enough heat to boil all the sample of water.
a) Heating from 84.7 °C to 100 °C:
This is calculated using the formula:
Q₁ = m * C * ΔT
Where Q₁ is the amount of heat, m is the mass of the sample, C is the specific heat of water and ΔT is the temperature change. We already know these values:
m = 41.1 g
C = 4.184 J/(g*°C)
ΔT = Tfinal - Tinitial = 100 °C - 84.7 °C
ΔT = 15.3 °C
Replacing these values we can get the amount of heat necessary for the first step:
Q₁ = m * C * ΔT
Q₁ = 41.1 g * 4.184 J/(g°C) * 15.3 °C
Q₁ = 2631 J
b) Boiling 41.1 g of water:
To find the amount of heat that we need to provide to the sample of water to completely boil it we can use this formula:
Q₂ = m * Cv
Where Cv is the latent heat of vaporization.
Cv = 2256 J/g
Q₂ = m * Cv
Q₂ = 41.1 g * 2256 J/g
Q₂ = 92721 J
c) Total amount of heat:
Qtotal = Q₁ + Q₂
Qtotal = 2631 J + 92721 J
Qtotal = 95352 J = 95400 J
Qtotal = 95.4 kJ
Answer: The amount of heat needed to boil the sample of water is 95.4 kJ or 95400 J.
What is the mass of 6.02 x 1023 molecules of CO2?
Answers
ANSWER
EXPLANATION
Given that
The number o molecules is 6.02 x 10^23 molecules
Firstly, find the number of moles using the below formula
[tex]\text{ mole = }\frac{\text{ number of molecules}}{\text{ Avogadro's number}}[/tex]Recall, that the Avogadro's number is 6.02 x 10^23
[tex]\begin{gathered} \text{ mole = }\frac{6.02\text{ }\times\text{ 10}^{22}}{6.02\text{ }\times\text{ 10}^{23}} \\ \text{ mole = 1} \end{gathered}[/tex]Secondly, find the mass of CO2 using the formula below
[tex]\text{ mole = }\frac{mass}{\text{ molar mass}}[/tex]Recall, that the molar mass of CO2 is given as 44.01 g/mol
[tex]\begin{gathered} \text{ mole = }\frac{\text{ mass}}{\text{ molar mass}} \\ \text{ mass = mole }\times\text{ molar mass} \\ \text{ mass = 1 }\times\text{ 44.01} \\ \text{ mass = 44.01 grams} \end{gathered}[/tex]Therefore, the mass of CO2 is 44 grams
Where do the following boxes on the right belong to?
Answers
According to the question, we are to categorize the definitions in the box as elements, compounds, or mixtures.
For ELEMENTS
• Since element,s consist of only one kind of atom,, hence they are ,substances made up of one type of particle (atoms or molecules)
• Since matter is anything that has mass and occupies space and is made up of substances called elements, hence, they are anything that has mass and volume
,• Cannot be broken into simpler kinds, of matter since elements are made of atoms.
For COMPOUNDS
• Compounds can only be separated by chemical means but not by physical means. Hence we can say that ,compounds cannot be separated by physical means, but can be chemically
• Consists of atoms of two or more different elements bonded together
,• Has properties unique to the element that makes it up
FOR MIXTURES
• Since the mixture consists of, two or more different elements ,and/or compounds physically, hence they can be separated by physical means.
• They can be separated into simpler types of matter by physical means and keeps the, properties of parts, that make it up.