Answers
Answer:
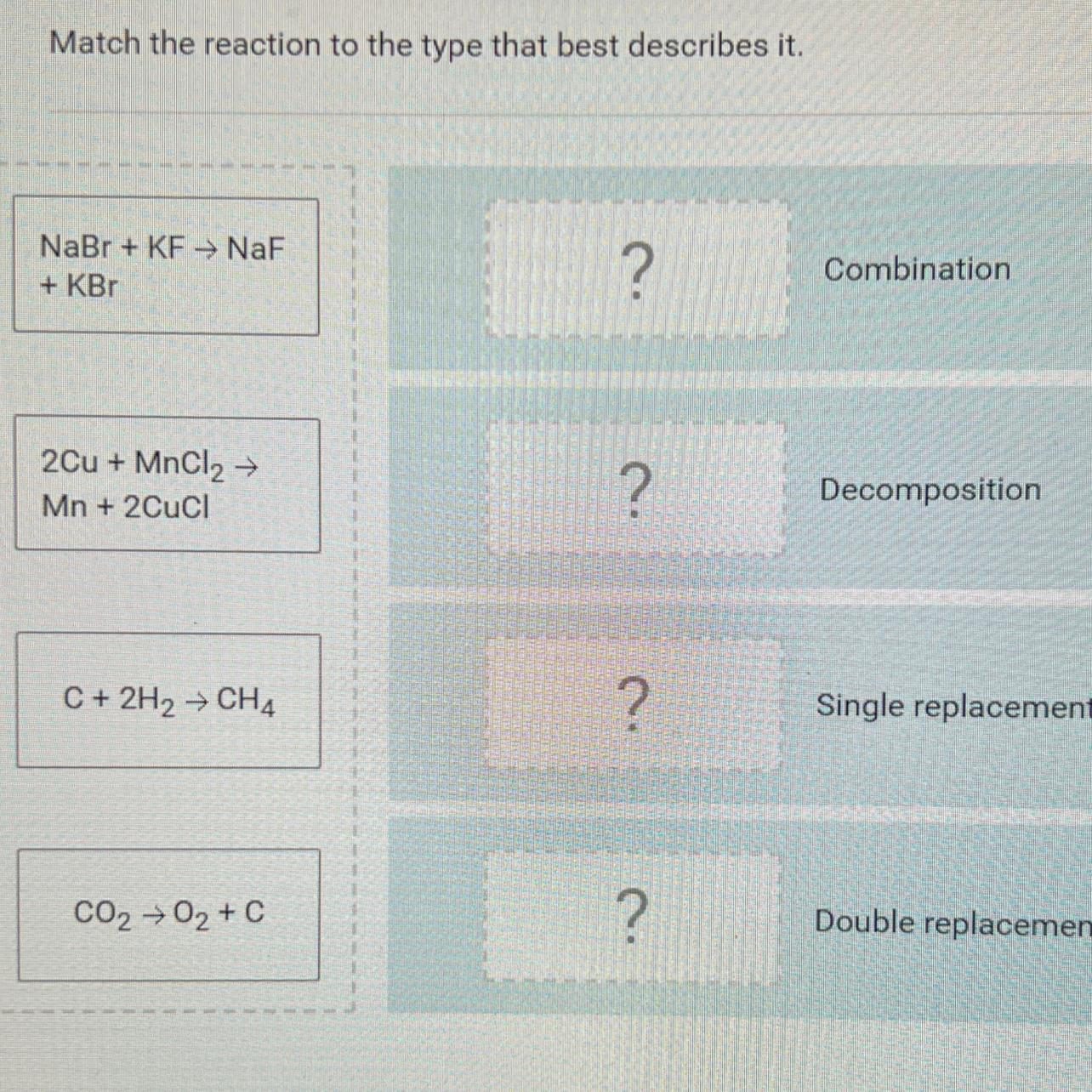
[tex]\begin{gathered} a:\text{ Double Displacement} \\ b:\text{ Single Replacement} \\ c:\text{ Combination} \\ d:\text{ Decomposition} \end{gathered}[/tex]Explanation:
Here, we want to get the type of reactions listed
We proceed to evaluate the reactions one after the other:
a) This is a double replacement reaction. It usually occurs when molecules exchange ions in the course of their reaction leading to replacement in a molecule by an ion or a group of ions in the other molecule
b) This is a single replacement reaction which in other words could be referred to as a displacement reaction. This occurs usually when a metallic solid reacts with a solution of the salt of less reactive metal
c) This is a combination reaction. It is usually useful in the laboratory and industrial synthesis of some molecules
d) This is a decomposition reaction. It occurs when a particular molecule splits into the constituent molecules that make it up
Related Questions
How many moles of O2 is in a 5.6 L sample of O2 measured at 250 kPa and 18°C?
Answers
Answer
578.66 mol
Explanation
Given:
Volume, V = 5.6 L
Temperature, T = 18° C = 18 + 273 = 291 K
Pressure, P = 250 kPa = 250000 Pa
Using the ideal gas equation, we can solve for the number of moles:
[tex]PV=\text{nRT}[/tex]Put the given values into the ideal gas equation to get the number of moles:
[tex]\begin{gathered} 250000\times5.6=n\times8.314\times291 \\ 1400000=2419.374n \\ \text{Divide both sides by 2419.374} \\ \frac{1400000}{2419.374}=\frac{2419.37n}{2419.374} \\ n=578.66\text{ mol} \end{gathered}[/tex]A 0.333 g-sample of antacid was dissolved in 40.00 mL of 0.135 MHCI solution, then back-titrated to the end-point with 9.28 mL of a0.0203 M NaOH solution.a) Calculate number of moles of acid in the original 40.00 mL of HCI?b) How many moles of base were used in the back titation of excessHCI?c) How many moles of excess HCII?a How many moles of HCl reacted with the antacid sample?
Answers
A) To find the number of moles of a compound, we can use the Molarity formula, which tells us that molarity is equal to the number of moles divided by the volume in liters.
M = n/V
Now in our question we have a few informations, the molarity and the volume
0.135M = n/0.04L
Therefore the number of moles will be:
n = 0.135M * 0.04L
n = 0.0054 moles or 5.4 * 10^-3 moles
B) Using the same molarity equation as it was used in letter A, we can find the number of moles:
M = n/V
0.0203M = n/0.00928
n = 0.000188 moles or 1.88 * 10^-4
C) Since the ratio of this reaction HCl + NaOH is 1:1, which means I need 1 mol of HCl to react with 1 mol of NaOH, we can easily find the excess by subtracting both numbers of moles:
Moles of HCl - Moles of HCl that reacted
0.0054 - 0.00018 = 0.0052 of excess of HCl or 5.2 * 10^-3
HCl + NaOH -> NaCl + H2O
Options for the first box: less then, equal to, greater than Options for the second box: is greatest, is least, equals standard pressure
Answers
Water boils when the vapor pressure is equal to the atmospheric pressure.
The atmospheric pressure is least at the top of the mountain.
Select the correct answer from each drop-down menu. How can you reduce air pollution arising from transportation?
Answers
Explanation:
When using public transport vehicles instead of private cars, we emit less pollution. For example in a bus more than 20 people travel, so the pollution per person is lower.
When we increase speed we usually consume more fuel, so avoiding accelerating rapidly we also reduce pollution.
Answer:
a) public transport vehicles.
b) accelerating rapidly.
Iron (III) oxide reacts with Carbon to form Iron and Carbon monoxide. How many moles of Iron can be formed from 125 KILOGRAMS of Iron (III) oxide?
Answers
For the complete reaction of 130 ml 0.140 mole ,130 ml of 0.14 moles is required.
What is moles?
mole Is the unit of amount of substance in the international system of unit.
For the complete reaction of 130 ml 0.140 M CO (NO3)2, 130 ml of 0.14 is required.
The reaction of Li2S with CO(NO3)2 will be:
Li2S +CO(NO3)2-->2LiNO3+COS
Accordingly, 1 mole of Li2S completely reacts with 1 mole of CO(NO3)2.
moles of CO(NO3)2 - Molarity Volume (L)
moles of = 0.140 M × 0.13 L
moles of CO(NO3)2 = 0.0182 moles.
The moles of Li2S reacts with CO(NO3)2 will be 0.0182 moles.
Volume of = Li2S= moles/molarity
Volume of = Li2S 0.0182/0.140
Volume of Li2S = 0.13 L
Volume of Li2S = 130 ml.
For the complete reaction of 130 ml 0.140 M , 130 ml of 0.14 is required.
To know more about moles
click-https://brainly.com/question/15356425
#SPJ1
Why different bonds might have diffrent bond strengths
Answers
In a covalent bond, one pair of electrons, two and three can be shared. Forming single, double, or triple bonds. The more electron pairs are shared, the stronger the bond will be.
At some temperature, an equilibrium mixture, in a 1.00-L container, involving the chemical system
PCl5(g) == PCl3(g) + Cl2(g)
is found to contain 2.01×1021 molecules of PCl5, 0.00377 mol of PCl3, and 0.519 g of Cl2.
Calculate the equilibrium constant (Keq expressed in terms of the molar concentrations) at this temperature.
Answers
The equilibrium constant Keq is the ratio of product of concentration of products in a reaction to that reactants. In the given reaction the equilibrium constant is 1.05 × 10²⁴.
What is equilibrium constant?The equilibrium constant in a reaction is the ratio of product of molar concentrations of products to the product of molar concentration of the reactants in the reaction.
In the given reaction, the product is PCl₅ and reactants are Cl₂ and PCl₃. Thus the expression for Keq is written as:
[tex]Keq = \frac{[PCl_{5}]}{[PCl_{3}][Cl_{2}]}[/tex]
Apply the number of moles and volume of all the reactants and products in this expression.
Keq = (2.01 × 10²¹molecules /1 L ) [(0.12/1L) (0.15/ 1L)]
= 1.05 × 10²⁴
Hence, the equilibrium constant Keq is 1.05 × 10²⁴.
To find more on equilibrium constant, refer here:
http://brainly.com/question/10038290
#SPJ1
What is the name of the molecule below?HHOA. MethaneOB. MetheneO C. EthyneOD. EtheneC=CHH
Answers
In this case, we have a two carbon molecule, the prefix for this type of molecule is Et
Since this compound has a double bond, the suffix will be ene
Therefore we have in this question, the molecule Ethene, letter D
I need assistance on number 5 including all parts of number 5 thank you !
Answers
5a) Propyne is a member of the alkyne group with the formula C3H4. The compound consists of 3 carbon atoms and 4 hydrogen atoms. The expanded form of the compound is as shown below;
b) For the skeletal structure of 2, 2 - dimethyl butane, the carbon and hydrogen atoms are hidden. The structure will only be written in form of lines as shown:
c) The molecular formula for benzene is C6H6. It is cyclic in nature with a total of 6 carbon atoms and 6 hydrogen atoms. The structure of the atom is as shown below;
d) Heptane is an alkane compound with the molecular formula C7H16. The condensed formula do not contain any lines or angles. They are written in term of their hydrogen and carbon compounds.
[tex]CH_3CH_2CH_2CH_2CH_2CH_2CH_3[/tex]Determine the pressure change when a constant volume of gas at 1.00 atm is heated from 20.0°c to 30.0° c show your work how to solve
Answers
In order to solve this question, we will be using the Gay-Lussac's gas Law, which is an experimental gas law that shows the relationship between temperature and pressure for a gas, the formula is:
P1/T1 = P2/T2
We have:
P1 = 1.00 atm
T1 = 20.0°C, but we need it Kelvin, therefore, 293 K
P2 = ?
T2 = 30°C, or 303 K
Now we add these values into the formula:
1/293 = P2/303
P2 = 1.03 atm is the new pressure
Write 5 sentences about voltaic cells.
Answers
ANSWER
EXPLANATION
Voltaic cell is a type of an electrochemical cell that uses chemical reaction to generate electrical energy
In voltaic cell, the oxidation process occur at the anode while reduction processes occur at the cathode.
2.60 moles CO and 2.60 moles H2O are placed in a 2.00L container at 690 °C (Keg=10.0).Calculate all equilibrium concentrations in the following reaction:
Answers
Answer:
considering the information given, the concentrations at equilbrium are:
CO(g) = 1.30 M
H2O(g) = 1.30 M
CO2(g) = 4.11 M
H2(g) = 4.11 M
Explanation:
The question requires us to calculate the concentration of CO, H2O, CO2 and H2, given the following information:
- number of moles of CO = 2.60 mol
- number of moles of H2O = 2.60 mol
- volume of container = 2.00 L
- temperature = 690°C
- equilibrium constant = 10.0
- balanced chemical equation:
[tex]CO_{(g)}+H_2O_{(g)}\rightleftarrows CO_{2(g)}+H_{2(g)}[/tex]Since the number of moles of CO and H2O were provided, as well as the volume of the container, we can calculate the concentration of this gases in moles per liter:
[tex]\begin{gathered} [CO\rbrack=\frac{2.60mol}{2.00L}=1.30mol/L=1.30M \\ \\ [H_2O\rbrack=\frac{2.60mol}{2.00L}=1.30M \end{gathered}[/tex]Also, since we have the equilibrium constant for this reaction and the concetrations of CO2 and H2 at equilibrium will be the same (they have the same stoichiometric coefficient), we can calculate the concentration of the products using the expression for Keq.
First, let's write the Keq expression for this reaction:
[tex]K_{eq}=\frac{[CO_2\rbrack\times[H_2\rbrack}{[CO\rbrack\times[H_2O\rbrack}[/tex]Considering,
[CO2] = [H2] = x
and rearranging this equation to calculate x:
[tex]K_{eq}\times[CO\rbrack\times[H_2O\rbrack=x^2\rightarrow x=\sqrt[2]{K_{eq}\times[CO\rbrack\times[H_2O\rbrack}[/tex]Now, applying the value of Keq given by the question, and the values of concentration calculated previously, we'll have:
[tex]x=\sqrt[2]{10.0\times1.30M\times1.30M}\rightarrow x=4.11M^[/tex]Therefore, considering the information given, the concentrations at equilbrium are:
CO(g) = 1.30 M
H2O(g) = 1.30 M
CO2(g) = 4.11 M
H2(g) = 4.11 M
how many molecules of p2o5 are there in 7.32 moles
Answers
In order to answer this question, we will need to Avogadro's number, which represents the number of atoms or molecules within 1 mol of a compound, this number is equal to 6.02*10^23 atoms or molecules, now we want to know how many molecules there are in 7.32 moles, we will do that by performing the following calculation:
1 mol = 6.02*10^23 molecules
7.32 moles = x molecules
x = 4.41*10^24 molecules
At sea level, there are approximately 2.6 × 1025 molecules m–3 of the atmosphere. There are 5.20 × 1021 molecules m–3 of one of the gases making up the atmosphere. What is the concentration of this gas as a proportion of the total number of molecules in the atmosphere, expressed in parts per million (ppm)?
Answers
Answer:
[tex]200\text{ ppm}[/tex]Explanation:
Here, we want to calculate the concentration of the gas in ppm
What we have to do here is to divide the number of molecules of the gas by the total number of molecules in the atmosphere, after which we can convert it to ppm
Mathematically, we have that as:
[tex]\frac{5.20\text{ }\times\text{ 10}^{21}}{2.6\times10^{25}}\text{ = 0.0002}[/tex]Now, we have to convert to ppm
To convert to ppm, we simply have to multiply the given ratio by 1,000,000
We have this as:
[tex]0.0002\text{ }\times\text{ 1000000 = 200 ppm}[/tex]How much motion energy does an 0.5-kg hockey puck sliding across the ice rink at 4 m/s have?
Answers
Answer:
A hockey puck is given an initial speed of 5.3 m/s . If the coefficient of kinetic friction between the puck and the ice is 0.05, how far does the puck slide before coming to rest? Solve this problem using conservation of energy.
Explanation:
This is easy to do and it is very cool how it works.
Force here is what converts the kinetic energy to heat by friction and slows the puck
fu = coefficient of Kinetic friction
g = is the acceleration of gravity
Fu*g = a.
So friction force on the puck is the acceleration of gravity times the coefficient of kinetic friction and the acceleration force is made negative here because it is slowing down the puck. .
Puck friction to negative acceleration
fu=0.05
g = -9.81m/s^2
so
a = 0.05 * -9.81m/s^2 = -0.4905 m/s^2
For the moving puck
Velocity = v = u + (a*t) | u=initial velocity
So
Time = t = (v-u)/a
Distance = d = t *(v + u)/2
So
Distance d= ((v-u)/a) * ((v+u)/2)
This becomes d = (v-u)(v+u)/2a
Plugging in the numbers
d= (0 - 5.3)(0 + 5.3)/(2*-0.49.5) =28.63 meters
what is the logic behind drawing lewis fomrulas for like harder and weird compounds like how can you figure it out easy.harder compounds like ch3och3
Answers
Answer:
The logic behind drawing Lewis formulas is to use the valence electrons of the elements and complete the Octet rule.
Explanation:
The logic behind drawing Lewis formulas is to use the valence electrons of the elements and complete the Octet rule.
Valence electrons are the electrons of an atom that are located in the last shell. We represent the valence electrons using dots between covalent bonds.
The Octet rule says that an element loses or gains electrons in order to achieve the electron configuration of the nearest noble gas, it means that it will try to have 8 electrons in the last shell.
For example: CH3OCH3
1st) It is necessary to find the total number of valence electrons. We can find the electrons of valence of each atom in the Periodic Table, and then we have to multiply it by the number of atoms in the molecule:
- Total number of valence electrons of C: 4e * 2 = 8e
- Total number of valence electrons of H: 1e * 6 = 6e
- Total number of valence electrons of O: 6e * 1 = 6e
Total number of valence electrons of CH3OCH3 = 20e (8 + 6 + 6 = 20)
2nd) Now we have to start drawing with the least electronegative atom in the center. In this case, we have to draw the O in the center (even though it is not the least electronegative atom in the molecule), because it is the only one that has only 1 atom in this molecule, and hydrogen atoms always go on the outside:
We have to start drawing the electrons, trying to complete the octets on the outside atoms, in this case, hydrogen, then carbon, and finally oxygen.
Hydrogen atom only needs 2 valence electrons to complete the last sheel.
calculate the mass in g of CaCl2 required to make 230 mL of a 75% (w/v) CaCl2 solution
Answers
The concentration of CaCl₂ is 75 % W/V. This means 75 g of the solute in 100 ml solution. Therefore, mass of calcium chloride required in 230 ml of this solution is 172.5 g.
What is mass by volume percentage?Mass by volume percentage of a solute is term used to represent the concentration of the solution. % W/V of a solution is the mass of solute in grams per 100 ml of the solution.
It is given that the mass by volume percentage of the calcium chloride solution is 75% (w/v). Thus 75 g of calcium chloride is present in 100 ml of the solution.
Therefore, the mass of calcium chloride in 230 ml of this solution is calculated as follows:
mass of solute = (230 ml ×75 g) /100 ml
= 172.5 g
Hence, the mass of calcium chloride is 172.5 g.
To find more on mass by volume percent, refer here:
https://brainly.com/question/15461083
#SPJ1
Balance the following equation using the change in oxidation numbered method. Ag +H2 S +O2 —> Ag2 S + H2O
Answers
To balance an equation with the oxidation numbered method we must write the oxidation states of the elements that participate in the reaction.
[tex]Ag_^0+H_2^{+1}S^{-2}+O_2^0\rightarrow Ag_2^{+1}S^{-2}+H_2^{+1}O^{-2}[/tex]Once we have the oxidation states we must identify how they change from reactants to products. Thus we identify which are oxidized and which are reduced. If its oxidation state increases it is because the element is oxidized, if it decreases it will have been reduced.
We see that the elements that change their oxidation state are silver, Ag and Oxygen. We have that:
Agas is contained in a thick-walledballoon. When the pressure changesfrom 100 kPa to 90.0 kPa, thevolume changes from 2.50 L to3.75 L and the temperature changesfrom 303 K toK.
Answers
In this question, we have a situation where all 3 known gas laws are being used in what is called the Combined gas law, and the formula for this law is:
P1V1/T1 = P2V2/T2
We have:
P1 = 100 kPa, or 0.987 atm
V1 = 2.50 Lites
T1 = 303 K
P2 = 90 kPa, or 0.888 atm
V2 = 3.75 L
T2 = ?
Now we add these values into the formula:
0.987 * 2.50/303 = 0.888 * 3.75/T2
2.47/303 = 3.33/T2
0.00815 = 3.33/T2
T2 = 408 K is the new temperature
Calculate the concentration of H3O + ions in a solution of NaoH whose concentration is 0.62M at 25 ° C.
Answers
The pH of this solution is 0.207
Hello
To solve this question, we need to use the formula of solving pH of a solution from hydrogen or hydronium ion.
[tex]\begin{gathered} pH=-\text{log\lbrack{}H}_3O^+\rbrack \\ \end{gathered}[/tex]Now, we would proceed to substitute the given value and solve for it.
[tex]\begin{gathered} pH=-\log \lbrack0.62\rbrack \\ pH=0.207 \end{gathered}[/tex]NB: we did not use the value of temperature because it is not needed in this solution.
From the calculation above, the pH of this solution is 0.207
Use the equation below to answer (What do you need to do first???)C3H8, + 02 →CO2,+ H2O1. How many moles of CO2, are produced for each mole of C3H8 used?2. If you start with 362 grams of C3H8 how many grams of H2O will be produced?
Answers
a) To get the number of moles of CO2 that are produced for each mole of C3H8 used, we will use the stochiometry ratio from the balanced chemical reaction.
The balanced chemical reaction that resulted from the combustion of propane gas is given as:
[tex]C_3H_8+5O2\to3CO_2+4H_2O[/tex]From the balanced reaction, you can see based on stoichiometry that for each mole of propane that reacted, 3 moles of CO2 was produced.
b) If you start with 362 grams of C3H8, the number of moles of propane will be expressed according to the formula:
[tex]\text{moles of C}_3H_8=\frac{Mass}{Mola\text{r mass}}\text{ }[/tex]Given the following parameters:
Mass of propane gas = 362 grams
Molar mass of propane = (3*12) + (1*8) = 36 + 8 = 44g/mol
[tex]\begin{gathered} \text{Moles of C}_3H_8=\frac{362}{44} \\ \text{Moles of C}_3H_8=8.227moles \end{gathered}[/tex]From stoichiometry, you can see that 1 mole of C3H8 produces 4 moles of H2O, hence the moles of H2O that will be produced is given as:
[tex]\begin{gathered} \text{Moles of H}_2O=4\times8.227 \\ \text{Moles of H}_2O\approx32.91\text{ moles} \end{gathered}[/tex]Get the mass of H2O that was produced using the formula:
[tex]\begin{gathered} \text{Mass of H}_2O=moles\text{ }\times molar\text{ mass} \\ \text{Mass of H}_2O=32.91\cancel{\text{moles}}\times\frac{18g}{\cancel{\text{mol}}} \\ \text{Mass of H}_2O=32.91\times18g \\ \text{Mass of H}_2O=592.38\text{grams} \end{gathered}[/tex]Therefore if you start with 362 grams of C3H8, 592.38grams of H2O will be produced
What conditions are needed for large storm formation?
Answers
Answer:
Lifting, instability, and moist/humid air
Explanation:
The water is released from the air, forming storm clouds, while humid air is flowing upward at a zone of low pressure over warm ocean water. The air in a hurricane rotates as it rises.
If Mr. Ken used 1.8 moles of sodium in the chemical reaction how many grams of sodium would he have used
Answers
We can always convert mass (g) to moles by using the molar mass of the substance, which can be found in the periodic table. In this case, we will use the molar mass of sodium (Na), which is 23g/mole, i.e., each 23g of sodium corresponds to 1 mole.
Since we want to find the mass of 1.8 moles of Na, we can set the following proportion:
[tex]\begin{gathered} 23g\text{ of Na ----- 1 mole} \\ x\text{g of Na ------ 1.8 moles} \\ \\ x=\frac{1.8\times23}{1}=41.4g \end{gathered}[/tex]So, by using 1.8 moles of Na, we are using 41.4 g of sodium (Na).
if a compounds of calcium oxide has a mass of 5.45 grams what would be the number of moles for this mass
Answers
To answer this question, we have to use calcium oxide molar mass. It is 56.1g/mol, which means that 1 mol of this compound is 56.1 grams. Use this to find how many moles are there in 5.45grams of calcium oxide:
[tex]5.45gCaO\cdot\frac{1molCaO}{56.1gCaO}=0.097molCaO[/tex]It means that for this mass, there are 0.097 moles of CaO.
Mass (M) will measured in which unit?O grams (g)O kelvin (k)O milliliters(ml)O Seconds (s)
Answers
According to these options presented here, we can see that:
Kelvin is for temperature
Milliliters are for volume
Seconds is for time
So, the answer for mass is grams (g). Therefore, we measure the mass in grams.
Answer: grams (g)
You have 2.7 x10^24 particles of C3H8:. How many grams are present?
Answers
Step 1 - Understanding molar mass
As we have previously seen, one mole corresponds to 6*10^23 particles.
When we weight one mole of something, we obtain what is called the molar mass: the mass of one mole of particles.
Each element has his own molar mass, which can be found in the periodic table. We can use them to calculate the molar mass of a molecule.
Let's see an example. Water is H2O. Looking at a periodic table, we find the molar masses 1 g/mol for H and 16 g/mol for O. Therefore, the molar mass of water will be:
[tex]M_{H2O}\rightarrow2H+1O=2\times1+1\times16=18\text{ g/mol}[/tex]Which means that 1 mole of water weights 18g.
Step 2 - Solving the exercise
Let's start by calculating the molar mass of C3H8. The molar mass for C is 12 g/molj, so:
[tex]M_{C3H8}=3C+8H=3\times12+8\times1=44\text{ g/mol}[/tex]44g corresponds thus to one mol of C3H8. Since one mole is 6.10^23 particles, we can set the following proportion:
[tex]\begin{gathered} 6.10^{23^\text{ }}particles\text{ of C3H8 ------ 44g} \\ 2.7\times10^{24}\text{ particles of C3H8 ----- x} \\ \\ x=\frac{2.7\times10^{24}\times44}{6.10^{23}}=\frac{1188}{6}=198g \\ \end{gathered}[/tex]Answer: 198g of C3H8 are present.
What is the molarity of a solution after 14.38 mL of 1.950 M NaCI solution is distilled into a total of 533.98 mL solution?
Answers
To answer this question we have to use the rule of dilutions:
[tex]C1V1=C2V2[/tex]Where C1 and C2 are the initial and final concentrations and V1 and V2 are the initial and final volumes, respectively. In this case we have to find C2 and the other values are given:
[tex]\begin{gathered} C2=\frac{C1V1}{V2} \\ C2=\frac{14.38mL\cdot1.950M}{533.98mL} \\ C2=0.052M \end{gathered}[/tex]It means that the answer is 0.052M.
13. Choose the geologic formation that fits the description: the world's longest mountain chain.
Answers
The mid-ocean ridge would be the longest and largest mountainous region. With a circumference of 40,389 miles, it genuinely is a world landmark.
The water covers around 90% of the mid-ocean ridge system. The world is crisscrossed by a system of mountains as well as valleys that resembles the seams in a baseball. Tectonic plate movement on Earth is what creates it.
Mountain ranges like the Rockies had also often grown and fallen over the evolution of our planet. Whenever two continents clash, mountains are created. Due to their identical weight as well as diameter, neither plate would bury beneath the other.
In a process known as plate tectonics, sections of such Earth's mantle, known as plates, collide with one another as well as buckle up like such a rear bumper in a frontal collision to build the world's largest mountain ranges.
To know more about mountain chain
https://brainly.com/question/29276761
#SPJ1
A 22 g sample of krypton exerts a pressure of140 mTorr at 40°C. What is the volume ofthe container?Answer in units of L
Answers
Answer
Volume = 36669.3 L
Explanation
Given:
Mass of krypton = 22 g
Pressure = 140 mTorr = 0.14 Torr
Temperature = 40°C = 313 K
Required: Volume
We know:
Molar mass of krypton = 83,798 g/mol
R constant in torr = 62.363577 L.Torr/K/mol
Solution:
Step 1: Find the number of moles of krypton
n = m/M where n is the number of moles, m is the mass and M is the molar mass
n = 22 g/83,798g/mol
n = 0.263 mol
Step 2: Find the volume using the ideal gas law
PV = nRT
V = nRT/P
V = (0.263 mol x 62.363577 L.Torr/K/mol x 313 K)/0.14 torr
V = 36669.3 L
Why is the timing of tides predictable?
The Earth's global winds are predictable.
The Moon's orbit around Earth is predictable.
The ocean water's density is predictable.
The Earth's rotation on its tilted axis is predictable.
Answers
The timing of the tide is predictable by the moon's orbit around the earth is predictable
Tides are the periodic rise and fall of surface water caused by the gravitational force of the moon and sun, as well as the rotation of the earth. The movements of the solar system that influence the tides are predictable, so changes in tide height and time are predictable.
Tides can be predicted accurately and far in advance. Tides are caused by the orbital relationships between the Earth, the Moon, and the Sun. These relationships are well understood, and the positions of the celestial bodies can be predicted very accurately in the future.
To learn more about tides visit
https://brainly.com/question/9493666
#SPJ1