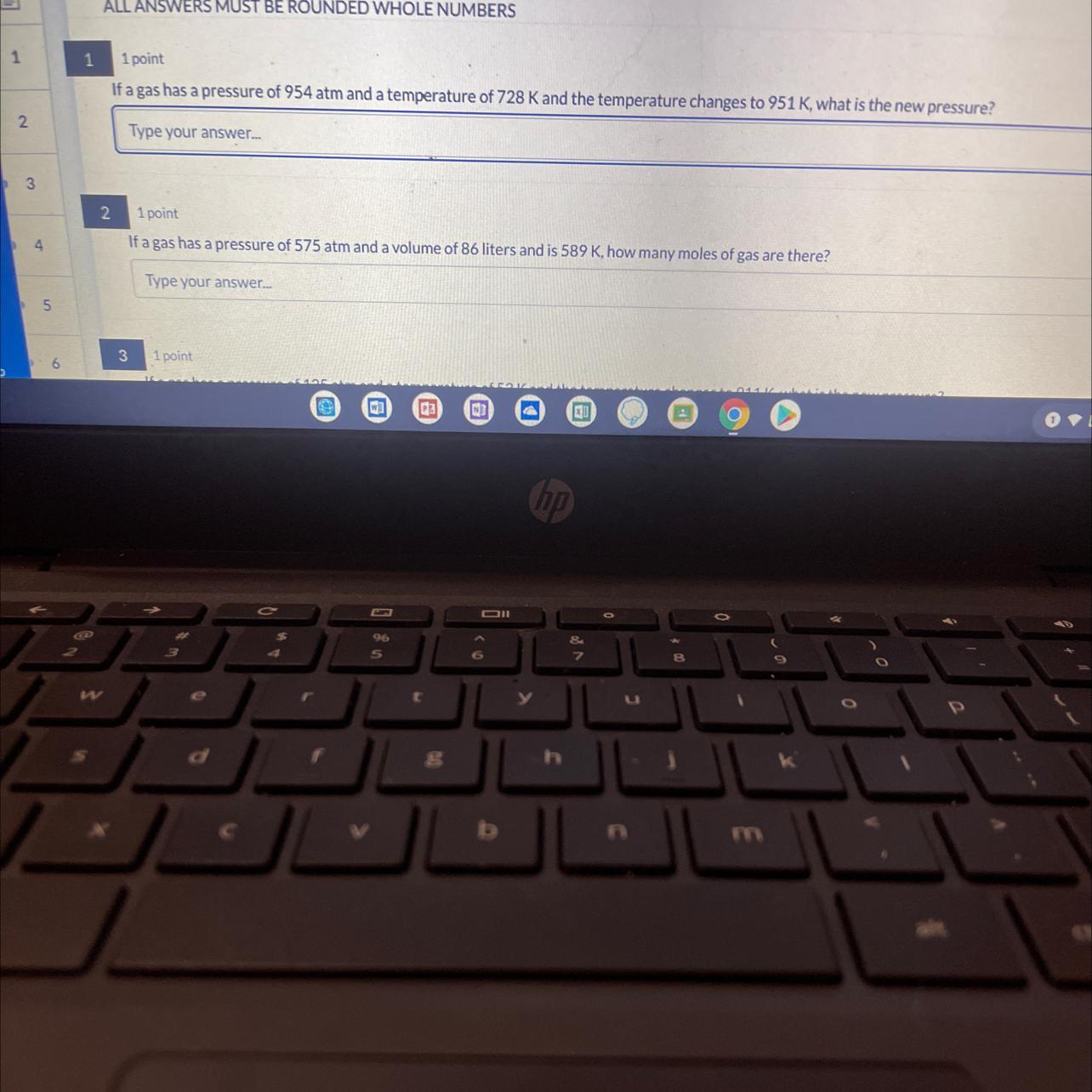
Answers
We have a change of pressure and temperature. To solve and find the new pressure we will assume that the volume and moles of the gas remain constant, therefore we can apply Gay-Lussac's law which tells us:
[tex]\frac{P_1}{T_1}=\frac{P_2}{T_2}[/tex]Where,
P1 is the initial pressure of the gas, 954atm
T1 is the temperature of the gas, 728K
T2 is the final temperature, 951K
P2 is the final pressure, =?
We clear P2 and replace the known data:
[tex]\begin{gathered} P_2=\frac{P_1}{T_1}\times T_2 \\ P_2=\frac{954atm}{728K}\times951K=1246atm \end{gathered}[/tex]Answer: The new pressure will be 1246atm
Related Questions
What mass of oxygen will be produced from the decomposition of 25.5g of potassium chlorate?
Answers
Answer
9.987 grams of O2
Explanation
Given:
Mass of potassium chlorate that decomposed = 25.5 g
What to find:
The mass of oxygen produced.
Solution:
The first step is to write the balanced chemical equation for the reaction.
2KClO₃ → 2KCl + 3O₂
From the equation; 2 moles of KClO₃ produced 3 moles of O₂
1 mole KClO₃ = 122.55 g/mol and 1 mole O₂ = 31.998 g/mol
This implies (2 mol x 122.55 g/mol) = 245.1 g of KClO₃ produced (3 mol x 31.998 g/mol) = 95.994 g of O₂
Therefore, 25.5 g of KClO₃ will produce
[tex]\frac{25.5\text{ }g\text{ }KClO_3}{245.1\text{ }g\text{ }KClO_3}\times95.994\text{ }g\text{ }O_2=9.987\text{ }g\text{ }O_2[/tex]Therefore, the mass of oxygen produced is 9.987 grams
My work gave me 12.3. Please walk me through how you would approach this problem so I can see where I went wrong.
Answers
In this question, we have HClO4 and KOH being mixed together and then a final pH being calculated, one way of doing this calculation is by finding out how many moles are being equivalent to each other and how many we have left divided by the total volume, so lets work on that:
HClO4 = 0.200 (volume) * 0.180 (concentration) = 0.036 moles
KOH = 0.270 ( concentration) * 0.150 (volume) = 0.0405
Total volume will be = 0.150+0.200 = 0.350 L
Now we can do the next calculation
(0.036 - 0.0405)/0.350
0.0128
This final value will be placed in the pH formula, which is:
pH = -log [H+]
pH = -log [0.0128]
pH = 1.89, letter D
How much heat energy would be needed to raise the temperature of a 15.0 g sample of iron [Specific Heat = 0.448 J/(g°C)] from 22.0°C to 100.0°C?1. 524J2. 11J3. 249J4. 8495. 201J
Answers
Answer:
[tex]524\text{ J}[/tex]Explanation:
Here, we want to get the amount of heat energy needed
Mathematically:
[tex]Q\text{ = mc}\Delta T[/tex]where:
Q is the amount of energy that we want to calculate
m is the mass of the iron sample which is 15 g
c is the specific heat capacity which is 0.448 J/g°C
ΔT is the change in temperature which is (100-22) = 78°C
Substituting the values, we have it as:
[tex]Q\text{ = 15 }\times\text{ 0.448 }\times\text{ 78 = 524.16 J}[/tex]Fill in the blank: Elements found in in the same vertical columns (Group 1A, 2A, etc) will share common properties. In the periodic table, the vertical columns of elements are called groups, or_________.
Answers
Answer
Elements found in in the same vertical columns (Group 1A, 2A, etc) will share common properties. In the periodic table, the vertical columns of elements are called groups, or families.
Explanation
The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. All the members of a family of elements have the same number of valence electrons and similar chemical properties. The horizontal rows on the periodic table are called periods.
Hence, elements found in in the same vertical columns (Group 1A, 2A, etc) will share common properties. In the periodic table, the vertical columns of elements are called groups, or families.
What does it mean if element A is higher than element B on the activity series?A.B will replace A in a single-replacement reaction.B.A will replace B in a combination reaction.C.A will replace B in a single-replacement reaction.D.B will replace A in a decomposition reaction.
Answers
Answer
C
Explanation
A higher ranked element in the activity series is more reactive in a single replacement reaction. We predict a single replacement reaction will occur when a less reactive element can be replaced by a more reactive element in a compound. Therefore since A is more reactive, it will replace B in a single replacement reaction.
How many moles of KNO3, should be used to prepare 0.70 L of a 0.560 M solution?
Answers
Answer:
0.392 moles of KNO3.
Explanation:
To find the moles of a solute based on the volume and concentration of a solution, we use the following formula:
[tex]Molarity\text{ \lparen M\rparen=}\frac{mole\text{s of solute}}{liter\text{s of solution}}=\frac{mo\text{l }}{L}.[/tex]The given data is: molarity = 0.560 M and volume (liters of solution) = 0.70 L. So, let's solve for 'moles of solute' and replace the values that we have. The solute in this case, would be KNO3:
[tex]\begin{gathered} mole\text{s of solute=Molarity \lparen M\rparen}\cdot liter\text{s of solution} \\ mole\text{s of KNO}_3\text{=0.560M}\cdot0.70\text{ L = 0.392 moles KNO}_3. \end{gathered}[/tex]We're going to use 0.392 moles of KNO3 to prepare 0.70 L of a 0.560 M solution.
identifying reaction types ZN+AgNO3
Answers
The type of reaction involved in the illustration is a single displacement reaction.
What are displacement reactions?Displacement reactions are chemical reactions in which an atom displaces another atom from a participating species of the reaction. Displacement reactions could be:
Single displacement reaction: here, an atom displaces another atom from a compound participating in the reaction. The reaction follows the example: A + BC ---> AB + C. Atom A has displaced atom C from the compound BC.Double displacement reaction: Here, radicals of compounds displace one another. In other words, two compounds exchange radicals to form two new compounds entirely, such that: AB + CD --> AC + BD.When you consider this reaction: [tex]Zn + AgNO_3 -- > ZnNO_3 + Ag[/tex]. Zn displaced Ag from the compound, [tex]AgNO_3[/tex]. Thus, it is a single displacement reaction.
More on displacement reactions can be found here: https://brainly.com/question/29307794
#SPJ1
Which of the following chemical equations violates the law of conservation of mass?
Answers
The law of conservation of mass states that mass is not created nor destroyed, it is just transforms.
A chemical reaction satisfies this law if the same amount of each element is present in both sides of the equation.
By looking at the choices, it can be observed that in the third choices, 1 sulfate ion is reacting and 2 sulfate ions are being produced. This violates the law of conservation of mass because if 1 sulfate ion reacts, 1 sulfate ion must be produced.
It means that the correct answer is the third choice.
A closed container contains a volume V of gas, composed of a mixture of 2 moles of O2 and 3 moles of CO2. The total pressure inside the container is 900 mm Hg. The container is at a temperature of 37⁰C? 1. Calculate PO2 and PCO2, the partial pressures of O2 and CO2 in the gas mixture. 2. Calculate the density of the gas mixture. 3. A quantity of water is introduced into the container, keeping the same quantity of gas, as before, above the water. The container is fitted with a piston, which is positioned so that the gaseous phase occupies a volume V′ and the total pressure of this gaseous phase above the water is maintained at 900 mm Hg. what is the difference between the gaseous phases in the two situations?
Answers
We have a closed container, so the number of moles remains constant.
Now, the total pressure is equal to the sum of the partial pressures. And the partial pressure of a gas will depend on its molar fraction, that is, the moles of the gas over the total moles. So the partial pressure is defined as:
[tex]P_i=\frac{n_i}{n_T}P_T[/tex]Where,
Pi is the partial pressure of the gas
ni, are the moles of the corresponding gas
nT, are the total moles
Pt is the total pressure.
1. Partial pressures of O2 and CO2
[tex]\begin{gathered} P_{O2}=\frac{2molO_2}{2molO_2+3molCO_2}\times900mmHg \\ P_{O2}=360mmHg \end{gathered}[/tex][tex]\begin{gathered} P_{CO2}=\frac{3molCO_2}{2molO_2+3molCO_2}\times900mmHg \\ P_{CO2}=540mmHg \end{gathered}[/tex]Answer: PO2=360mmHg
PCO2=540mmHg
2. Now, the density will depend of the number of moles and the volume. We can calculate the volume of the gases with the ideal gas equation that says:
[tex]\begin{gathered} PV=nRT \\ V=\frac{nRT}{P} \end{gathered}[/tex]For each gas we will have:
O2
PO2=340mmHg=0.45atm
T=37°C=310.15K
R is a constant = 0.08206 (atm.L)/(mol.K)
nO2=2mol
massO2=2mol x MolarMass = 2 mol x 31.998g/mol=63.996g
[tex]V_{O2}=\frac{2mol\times0.08206\frac{atm.L}{mol.K}\times310.15K}{0.45atm}=113.1L[/tex][tex]DensityO_2=\frac{Mass}{Volume}=\frac{63.996g}{113.1L}=0.57g/L[/tex]CO2
PCO2=540mmHg=0.71atm
T=37°C=310.15K
R is a constant = 0.08206 (atm.L)/(mol.K)
nCO2=3mol
massCO2=3mol x MolarMass = 3 mol x 44.01g/mol=132.03g
[tex]V_{CO2}=\frac{3mol\times0.08206\times\frac{atm}{mol}\times310.15K}{0.71atm}=107.5L[/tex][tex]DensityO_2=\frac{Mass}{Volume}=\frac{132.03g}{107.5L}=1.23g/L[/tex]Answer:
Density O2=0.57g/L
Density CO2 = 1.23g/L
3. In the second situation, what will happen is that the partial pressure of the gases will decrease, since due to the pressure exerted by the piston, part of the moles of gas will dissolve in the water. So in the gas phase we will have fewer moles of gas.
7.0 g of nitrogen is reacted with 5.0 g of hydrogen to produce ammonia according to the chemical equation shown below. Which one of the following statements is false? N2(g) + 3 H2(g) → 2 NH3(g)options:3.5 g of hydrogen are left overAll of them are falseHydrogen is the excess reactant.Nitrogen is the limiting reactant.The theoretical yield of ammonia is 15 g.
Answers
Step 1
N2(g) + 3 H2(g) → 2 NH3(g) (must be balanced)
The limiting reactant
Data needed:
The molar mass:
For N2) 28 g/mol
For H2) 2 g/mol
For NH3) 17 g/mol
Procedure:
28 g N2 ------ 3 x 2 g H2
7.0 g N2------- X = 1.5 g H2
According to this, for 7.0 g N2, 1.5 g H2 is needed to react, but 5.0 g is provided. Therefore, H2 is the excess, and N2 is the limiting reactant
1.5 g of H2 is reacted, so 5.0 g - 1.5 g= 3.5 g are leftover
------------------
Step 2
The theoretical yield.
Procedure:
28 g N2 ------ 2 x 17 g NH3
7.0 g N2------ X = 8.5 g NH3
Answer: The theoretical yield of ammonia is 15 g. FALSE
How many grams of water are produced in this reaction# 14
Answers
answer
13. the molar mass of O2 is 32 g/mol
conversion: 2 mols x 16 g/mol = 32 g
14. there are 2 mols of water produced and this equals:
2 mols x 18 g/mol = 36 g
An objects heat capacity depends on which of the following?
___ Mass (quantity) of the object
___ Shape
___ Magnitude of the temperature change
___ Composition
Answers
In which of the following would the particles be moving faster? A. ice at -20 °C B. water at 20°CC. steam at 110°CD. boiling water
Answers
ANSWER
Option C
EXPLANATION
The collision between particles of substance increases with an increase in the temperature.
Recall, that matter exists in three states which are;
solid
liquid
gas
The particles will move faster is gas because the intermolecular force binding the particles together is very weak and therefore, the collision between the particles are very fast
Therefore, the particles of steam at 110 degrees Celcius will move faster
Option C is correct
What are the raw materials used to make many synthetic substances? synthetic substances found in the home. List two products that contain each material.
Answers
Synthetic substances:
Synthetic fibers e.g. are made from organic synthetic high-molecular compounds and are made synthetically from raw materials such as petroleum oil or petrochemicals.
Until here the answer is: petroleum oil
At home, we can find synthetic fibers, ceramics, plastics, etc
We can find synthetic fibers (polyester, Rayon, Spandex, etc) in caps, raincoats, and ropes.
Plastic can be found in bottles, cups, etc.
I have attached an image with all of the information.
Answers
Explanation:
For reactants: BE 2CH2OH = 2*(3*414+351+460) = 4106kJ/mol
: BE 3O2 = 3*499 = 1497kJ/mol
For products: BE 2CO2 = 2*799 = 1598kJ/mol
: BE 4H2O = 4*2*460 = 3680kJ/mol
[tex]\begin{gathered} BE\text{ = Sum}_{reactants}\text{ - Sum}_{products} \\ \\ \text{ = 4106+1497-\lparen1598+3680\rparen} \\ \\ \text{ = 325kJ/mol} \end{gathered}[/tex]Answer:
Bond energy is 325kJ/mol
Hydrogen’s emission spectrum includes a line of violet light that has a frequency of 7.31 * 10^14 Hz. What is the energy (in joules) a photon of the violet light?
Answers
Explanation:
The energy of a photon of the violet light can be obtained as follows:
[tex]E\text{ = h x f}[/tex]E = energy
h = Plank's constant
f = frequency
-------
Data provided:
f = 7.31x10^14 Hz
--
Data needed:
h = 6.63x10^-34 Js
So, E = h x f = 6.63x10^-34 Js x 7.31x10^14 Hz = 4.85x10^-19 J
Answer: 4.84x10^-19 J (nearest value)
How many moles are there in 5.0grams of calcium bromide?
Answers
ANSWER
EXPLANATION
Given information
The mass of calcium bromide is 5.0 grams
Follow the steps below to find the mole of calcium bromide
Step1: Write the formula for calculating mole
[tex]\text{ Mole = }\frac{mass}{molar\text{ mass}}[/tex]Recall, that the molar mass of calcium bromide is 199.89 g/mol
Step 2: Substitute the given data into the formula in step 1
[tex]undefined[/tex]use the elements of the periodic table to help you identify the number of valence for each of these elements 1,2,3,4,5,6,7, or 8carbon (C) 1Chlorine (Cl) 2Neon (Ne) 3Calcium (Ca) 4Oxygen (O) 5 6 7 8
Answers
Answer:
Explanation:
Here, we want to get the valence electron number for each of the listed elements
The number of valence electrons is used to put elements into groups
Thus, an element will have 1 valence electron if it belongs to group 1
Now, let us get the valence electrons for each of the listed elements
Carbon C has 6 valence electrons
Chlorine Cl has 7 valence electrons
Neon Ne has 8 valence electrons
Calcium Ca has 2 valence electrons
Oxygen O has 6 valence electrons
Kindly note that we do not count the d-block when checking for the number of valence electrons. After the second group, we skip the next 10 (the transition metals) and count the next group, starting from Boron as 3, in that sequence
A student spends 4.5 hours studying for a test. How much time is this equivalent to in seconds? 16,200 seconds 270 seconds 6,480 seconds 2,700 seconds
Answers
Answer
16,200 seconds
Explanation
Conversion factor:
1 hour = 60 minutes
1 minute = 60 seconds
Therefore,
4.5 hours x 60 minutes/1 hour = 270 minutes
270 minutes x 60 seconds/1 minute = 16,200 seconds
a mixture of helium,nitrogen,and oxygen has a total pressure of 723 mmHg. the partial pressure of helium is 194 mmHg, and the partial pressure of nitrogen is 262 mmHg. what is the partial pressure (in mmHg) of oxygen in the mixture
Answers
Answer:
267 mmHg.
Explanation:
What is given?
Total pressure = 723 mmHg
Partial pressure of helium (He) = 194 mmHg.
Partial pressure of nitrogen (N) = 262 mmHg.
Step-by-step solution:
Let's see the formula of total pressure:
[tex]P_{TOTAL}=P_1+P_2+...[/tex]Let's write the formula to our context if we have 3 different gases:
[tex]P_{TOTAL}=P_{He}+P_{Ni}+P_O.[/tex]We want to find the partial pressure of oxygen (O), so let's solve for Po which is the unknown value in the formula, and replace the given data:
[tex]\begin{gathered} P_O=P_{TOTAL}-P_{He}-P_N \\ P_O=723\text{ mmHg-194 mmHg-262mmHg,} \\ P_O=267\text{ mmHg.} \end{gathered}[/tex]The partial pressure of oxygen is 267 mmHg.
Consider the density data in
the table at right.
Trial #
1
The sample is thought to be
silver, density = 10.49 g/cm³.
What is the range of the data? Average
2
3
Density
(g/cm³)
10.34
10.58
10.62
10.51
Answers
10.51 (g/cm³) is the Average Density of the given data.
Average Density = 10.34+10.58+10.62+10.51÷4
Average Density=42.05/4
Average Density=10.51
Density is defined as a material substance's mass per unit volume. Density is described by the equation d = M/V, where d stands for density, M for mass, and V for volume. Grams per cubic centimeter is the unit of measurement for density.
For instance, compared to Earth's density of 5.51 grams, water has a density of 1 grams per cubic centimeter. Another way to express density is in kilograms per cubic meter (in meter-kilogram-second or SI units)
It is easy to determine the relationship between mass and volume using density. For instance, the formula for determining a body's mass is M = Vd, while the formula for determining a body's volume is V = M/d.
To know more about density visit : https://brainly.com/question/2876257
#SPJ1
In the reaction below, what is the limiting reactant when 1.24 moles NH of reacts with 1.79 moles of NO?
Answers
To identify the limiting reactant, we use the coefficients of the reaction:
As you can see, we have 1.79 moles of NO, but we need 1.86 moles of NO according to the reaction. For this reason, NO is the limiting reactant since we need more than what we have.
Volume vs temp. Please help me find the final answer!
Answers
Given that the pressure in the whole process is constant, we could use the Charles's law:
[tex]\frac{V_1}{T_1}=\frac{V_2}{T_2}[/tex]Let's replace our values and solve the equation for T2:
[tex]\frac{623mL}{137C}=\frac{914mL}{T_2}\to T_2=\frac{137C\cdot914mL}{623mL}=201C[/tex]The temperature of the gas sample will be 201°C.
how much iodine (I2) in moles, should be added to water to produce 2.5L of solution with a molarity of 0.6M?
Answers
Molar concentration or Molarity is a way to measure the concentration of a solution after some solute has been added to the solvent, the formula for Molarity is:
M = n/V
where:
M = molar concentration
n = number of moles
V = volume of the solution in Liters
In the question we are given the molarity and volume, let's add these information to our formula
0.6 M = n/2.5 L
n = 1.5 moles of Iodine should be added
Which nonmetal element in group 17 was used long ago in war to gas and terminate the enemy?
Answers
In World War 1, Chlorine gas was used as a toxic, poisonous gas to harm and terminate enemies.
Describe how you can determine the total change in enthalpy and activation energy from the diagram, and if each is positive or negative.
Answers
In this diagram, we have a reaction occurring and 3 letters that represent Reactants, Activation Energy and Products. To determine which is which, first, we will almost always have the Activation Energy in the middle of the reaction, this is how much energy is required for the reaction to occur, therefore Activation Energy is represented by letter B
To determine the total change in enthalpy, we need to subtract the sum of all the enthalpy from the products, which is letter C, and the sum of all the enthalpy from the reactants, which is letter A
ΔH = C - A
In this graph, we have the energy increasing and more energy on the products side, this means that an input of energy was required, this is the description of an Endothermic reaction, therefore the sign will be Positive
We have seen that the behavior of gases can be described by 4 variablestemperature, pressure, volume and number of moles. Let's study the link between thepressure and the number of moles in a situation where the other two variablesremain constant. a) Using a few sentences, equations or diagrams, clearly explainsthe relationship between pressure and the number of moles. Your answer must include the notion ofcollisions to justify a pressure change. To help you answer, think ofcomplete the following sentence: "If the number of moles increases the pressure" (directly proportional or inversely proportional?) and thenjustify your choice.b) A balloon contains 4.0 moles of gas at a pressure of 1.6atm. you leave gasout of the balloon to end up with only 3.0 moles of gas. What's the newspressure inside the balloon, assuming that the volume and temperature have notnot changed. c) You need the identity of the gas to answer the question correctlyformer. True or false? No substantiation required.
Answers
Answer and Explanation
(a) At constant temperature and volume the pressure of a gas is directly proportional to the number of moles of gas.
P = n (RT/V) where P is the pressure, n is the number of moles, R is the gas constant, T is the temperature and V is the volume
So when the pressure in increased, the volume will be decreased, meaning there will be less space in the container, this will lead to more collisions inside a container, meaning, the number of moles will be increased.
[tex]\frac{P_1}{n_1}\text{ = }\frac{P_2}{n_2}[/tex](b) Given: initial number of moles (n1) = 4.0 moles
initial pressure (P1) = 1.6 atm
Final number of moles (n2) = 3.0 moles
Required: Final Pressure (P2)
solution
P1/n1 = P2/n2
P2 = P1*n2/n1
P2 = 1.6 atm*3.0 moles/4.0 moles
P2 = 1.2 atm
Which uses energy directly from the sun to produce electricity?A.Biomass powerB.Coal powerC.Solar powerD.Nuclear power
Answers
Explanation:
In solar power we use the radiation from the sun to produce electricity.
Answer: C. Solar power
The energy source that uses energy directly from the sun to produce electricity is solar power. The correct answer is option C.
Energy refers to the capacity or ability to do work or cause change. It is a scalar quantity that can be transferred or converted from one form to another, but it cannot be created or destroyed.
Solar power is generated by converting sunlight into electrical energy using solar panels or photovoltaic cells. These cells are made up of layers of silicon that absorb sunlight and convert it into an electrical current.
The energy produced by solar power is clean, renewable, and does not produce any harmful emissions or waste products.
In conclusion, solar power is the energy source that uses energy directly from the sun to produce electricity. Option C is the correct answer.
Learn more about energy here:
https://brainly.com/question/1932868
#SPJ6
What is the empirical formula of P4O6?
Answers
The empirical formulas refer to the formulas where the ratios are the simplest, while the molecular formula shows the number of each type of atom.
In this case, we have a molecular formula and we just have to simplify the number to get the empirical formula. Divide each number by 2.
[tex]\begin{gathered} \frac{4}{2}=2 \\ \frac{6}{2}=3 \end{gathered}[/tex]Therefore, the empirical formula is
[tex]P_2O_3[/tex]WHAT WILL BE THE PROPPER WAY FOR ANSWERING THIS QUESTION ASLIVING IN OKLAHOMA
Answers
The main way that could be told to do this is to actively show their presence in the campaign and get involved with the campaign with interest and enthusiasm.
Some other ways to tell the students of the region to do their part in a campaign are as follows:
Get involved in local campaigns and events.Write to your local representatives about the issues that matter to you.Make donations to local organizations working on the issue.Talk to your friends and family about the issue and why it's important to you.Contribute to the campaign by writing attractive slogans and making posters.Create awareness in your locality about the campaign.Arranging provisions properly for the participants taking part in the campaign.Propaganda about the campaign in social media and other modes of communication.These are some ways that could be told to the students of the region to take part in the campaign.
To know more about social awareness, click below:
https://brainly.com/question/28039248
#SPJ9