Answers
Answer
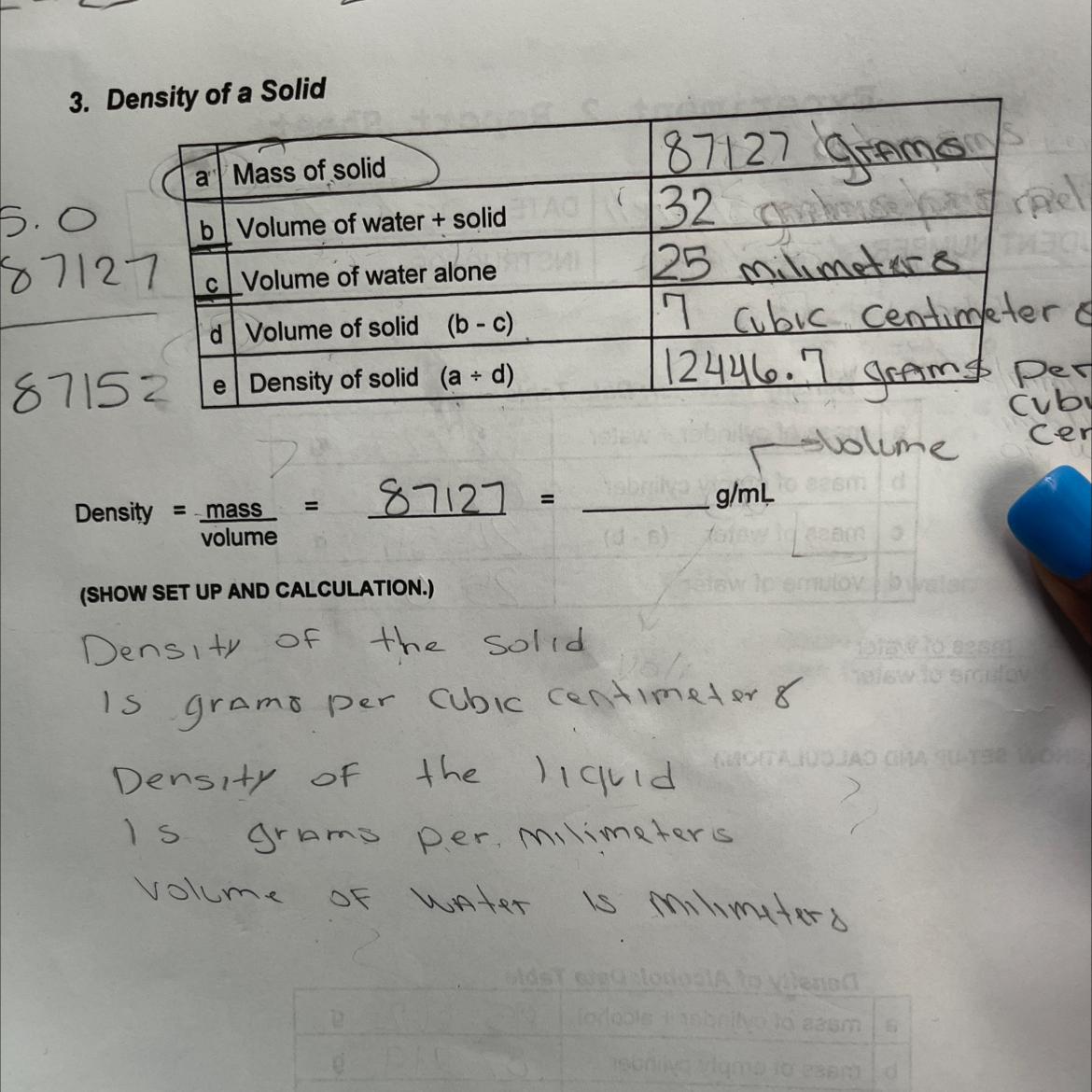
All the units were correct.
The unit of part b can either be any of milliliter or cubic centimeter
Density = 12446.71 g/mL
Explanation
All the units were correct.
Note:
1 milliliter = 1 cubic centimeter
g = grams
mL = milliLiter
The unit of part b can either be any of milliliter or cubic centimeter
The unit of the mass is g
The unit of the volume is mL
The unit of density is g/mL
The density of the solid = mass/volume = 87127/7 = 12446.71 g/mL
Related Questions
A chemist wants to make 7,837 mL of a solution with a concentration of 2.45 M.How many liters of a 6.85 M solution should be used to make this solution?
Answers
AnswerExplanation:
The liters of a 6.85 M solution that should be used to make the solution can be calculated using the dilution formula.
The formula for calculating dilutions is:
[tex]C_1V_1=C_2V_2[/tex]C₁ = 2.45 M
V₁ = 7837 mL = 7837/1000 = 7.837 L
C₂ = 6.85 M
V₂ = unknown
The unknown volume, V₂ is the required volume needed to make the solution.
Therefore, the V₂ can be calculated using the formula above as follows:
[tex]undefined[/tex]To reduce dangerous swelling in the brain, physician sometimes administer intravenous hypertonic saline, a 5.0%(w/v) Solution of salt (NaCI) In water. Calculate the volume of the solution that contains 0.50 g of NaCl. Be sure your answer has unit symbol and his rounded to the correct number of significant digits
Answers
Percent weight/volume (% w/v) is a way of expressing the concentration of a solutuon. The definition of it is:
% w/v = mass of solute /100 ml of solution
We have to find the volume of the 5.0 % (w/v) solution that contains 0.50 g of NaCl. So we are given these values:
Concentration % w/v = 5.0 %
mass of solute = 0.50 g
Since our solution is 5.0 % w/v Nacl, we know that it has 5.0 g NaCl in 100 ml of solution. So if we want to find the volume of solution that contains 0.50 g we can:
0.50 g of NaCl * 100 ml of solution/(5.0 g of NaCl) = 10. ml of solution.
10. mL of solution is the volume that contains 0.50 g of NaCl
Determine the mole fraction of methanol CH3OH and water is a solution prepared by dissolving 4.8 g of alcohol in 38 g of H2O.
Answers
Answer
Mole fraction of water = 0.066
Explanation
Given:
mass of methanol = 4.8 g
mass of water = 38 g
We know:
Molar mass of methanol = 32,04 g/mol
Molar mass of water = 18,01528 g/mol
Solution:
Step 1: Find the moles of methanol and water
n of methanol = m/M
n = 4.8g/32.04g/mol
n = 0.15 mol
n of water = 38g/18.01528
n = 2.11 mol
now mole fraction of methanol= mole of methanol/(mole of methanol + mole of water)
Mole fraction of methanol = 0.15mol/(0.15 mol + 2.11 mol)
Mole fraction of water = 0.066
You are preparing a solution from 750 L of a 3.0 M NaCl solution.
How many L of a 1.5 M NaCl solution could you produce by
diluting the original solution?
O 0.006 L
O 6L
Q 3000 L
Q 1500 L
Q 375 L
Answers
You are preparing a solution from 750 L of a 3.0 M NaCl solution then 1.5 M NaCl solution could you produce by diluting the original solution is 1500L
Solution is a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to what is called the limit of solubility.
Here given data is
Volume = V₁ = 750 L
M₁ = 3.0 M
M₂ = 1.5 M
V₂ = ?
We have to calculate L of a 1.5 M NaCl solution could you produce by diluting the original solution = ?
So the formula is
M₁V₁ = M₂V₂
3.0 M ×750 L = 1.5 M ×V₂
V₂ = 1500L
Know more about volume
https://brainly.com/question/3677716
#SPJ1
Nitrogen and hydrogen react to form ammonia according to the reaction show.
3H2(g) + N2 <- -> 2NH3.
What is the percent yield if 15.68g nitrogen and 10.74 g hydrogen react to form 5.24 g of ammonia?
Answers
The percent yield of the reaction if 15.68 g of nitrogen and 10.74 g of hydrogen react to form 5.24 g of ammonia would be 82.35%.
Stoichiometric problemFrom the balanced equation of the reaction below:
[tex]3H_2(g) + N_2 < - - > 2NH_3.[/tex]
The mole ratio of hydrogen to nitrogen is 3:1.
The molar mass of hydrogen is 2 g/mol
The molar mass of nitrogen is 28 g/mol
Recall that: mole = mass/molar mass
Mole of 10.74 g hydrogen = 10.74/2
= 5.37 mol
Mole of 15.68 nitrogen = 15.68/28
= 0.56 mol
This means hydrogen is in excess while nitrogen is the limiting reactant. The mole ratio of nitrogen and ammonia is 1:2. Thus, the equivalent mole of ammonia produced would be:
0.56 x 2 = 1.12 mol
Molar mass of ammonia = 17 g/mol
Mass of 1.12 mol ammonia = 1.12 x 17
= 19.04 grams
Percent yield of the reaction = 15.68/19.04 x 100
= 82.35%
In other words, the percent yield of the reaction is 82.35%.
More on stoichiometric problems can be found here: https://brainly.com/question/14465605
#SPJ1
How are the chlorate and chlorite ions different from each other?How are they similar to the sulfate and sulfite ions?
Answers
Explanation and Answer:
formula of Chlorite ion = ClO₂⁻
formula of Chlorate ion = ClO₃⁻
The chlorite ion contains two atoms of O, and the chlorate ion contains three atoms of O.
They are both cations and their charges are -1 but, the main difference between them is that the oxidation state of Cl in the chlorite ion is 3+ and the oxidation state of Cl in the chlorate ion is +5.
formula of sulfite ion = SO₃²⁻
formula of sulfate ion = SO₄²⁻
Sulfite and sulfate are both cations with charge -2. The oxidation state of S in the sulfite ion is +4 and the oxidation state of S in the sulfate ion is +6.
How are the chlorate and the chlorite ions similar to the sulfate and sulfite ions?
When naming a polyatomic ion the sufix is related to the oxidation state of the main element.
common positive oxidation states of Cl = +1 +3 +5 +7
common positive oxidation states of S = +4 +6
When the element has two main positive oxidation states (like S) , the sufix for the lower oxidation state is -ite (sulfite = +4) and the sufix for the greater oxidation that is usually more stable is -ate (sulfate = +6).
When the element has four main positive oxidation states (like Cl), the two in the middle are named in a similar way to the previous one. The lower oxidation state must have -ite as a sufix (chlorite = +3) and the sufix for the greater is -ate (chlorate = +5).
Ions are the charged chemical species. Chlorate and chloride are two different ions of chlorine which are represented as ClO₃⁻ and ClO₂⁻, respectively. These are similar to sulfate and sulfite ions in the difference in the charge present on them.
What are ions?
An ion is an atom or a molecule which carry a net electrical charge. The electric charge is a result of the valency of the atom.
Chlorite and chlorate are the two charged ions of chlorine and can be represented as:
Formula of Chlorite ion = ClO₂⁻
Formula of Chlorate ion = ClO₃⁻
The chlorite ion contains two atoms of O, whereas the chlorate ion contains three atoms of O. Both of these ions are anions as they carry a net negative charge with a charge of -1. The main difference between these two is the oxidation state of Cl in the chlorite ion which is +3 and the oxidation state of Cl in the chlorate ion is +5.
The sulfate and sulfite ions are the two charges species of sulfur which can be represented by:
formula of sulfite ion = SO₃²⁻
formula of sulfate ion = SO₄²⁻
Sulfite and sulfate are anions with a charge of -2. The oxidation state of sulfur in the sulfite ion is +4 and the oxidation state of sulfur in the sulfate ion is +6.
These are similar to chlorate and chloride ions in oxidation states which are: Oxidation state of Cl = +1, +3, +5, and +7 and Oxidation states of S = +4 and +6
Learn more about Ions here:
https://brainly.com/question/14982375
#SPJ2
What is the purpose in the procedure of chemistry lab: Titration?
Answers
1) Titration. This is a common laboratory method used to determine the unknown concentration of a substance.
2) We use a solution with a known concentration. This solution can be an acid (or a base). We fill a burette with this solution.
3) In a flask, we have the solution with unknown concentration and a known volume. It can be a base (or an acid).
4) Let the solution out of the burette and into the flask. We have to do this until the indicator changes color. Read the volume in the burette.
5) Finally, we have to calculate the concentration of the solution.
What is the mass of 6.02 x 1023 molecules of CO2?
Answers
ANSWER
EXPLANATION
Given that
The number o molecules is 6.02 x 10^23 molecules
Firstly, find the number of moles using the below formula
[tex]\text{ mole = }\frac{\text{ number of molecules}}{\text{ Avogadro's number}}[/tex]Recall, that the Avogadro's number is 6.02 x 10^23
[tex]\begin{gathered} \text{ mole = }\frac{6.02\text{ }\times\text{ 10}^{22}}{6.02\text{ }\times\text{ 10}^{23}} \\ \text{ mole = 1} \end{gathered}[/tex]Secondly, find the mass of CO2 using the formula below
[tex]\text{ mole = }\frac{mass}{\text{ molar mass}}[/tex]Recall, that the molar mass of CO2 is given as 44.01 g/mol
[tex]\begin{gathered} \text{ mole = }\frac{\text{ mass}}{\text{ molar mass}} \\ \text{ mass = mole }\times\text{ molar mass} \\ \text{ mass = 1 }\times\text{ 44.01} \\ \text{ mass = 44.01 grams} \end{gathered}[/tex]Therefore, the mass of CO2 is 44 grams
Q: Use the equation to calculate the following:2 NaOH(aq) + H1S04(aq)- Na2SO4(aq) + 2 H2O(/)(a) the moles of Na2SO4 produced from 3.6 mol H2SO4?
Answers
1) First, let's write the equation:
NaOH(aq)
Which of the following would likely be a non -conductor of electricity?a) An aqueous solution of magnesium sulfate (Epsom salts)b) Gasolinec) An aqueous solute of sodium carbonate (washing powder)d) Carbonated soft drinksExplain your answer.
Answers
This would be the Gasoline, letter B, since gasoline is a hydrocarbon and these compounds are formed with covalent bonds, they share electrons and not gain or lose, forming ions and then conducting electricity, as we can see in the other 3 options, they all form ions.
why do engineers come up with as many ideas to solve a problem as possible?
Answers
Engineers come up with as many ideas to solve a problem as possible is so as to choose the best which is applicable in helping people.
Who is an Engineer?This is referred to as an individual or a professional which employs the use of scientific knowledge in the construction and building of different types of machines and tools.
An engineer is someone who has a good analytic and problem solving skills and comes up with many ideas so as to be able to choose the best from the set of alternatives in helping people solve their problems or issues.
Read more about Engineer here https://brainly.com/question/17169621
#SPJ1
What volume of 1.00 M HCl in liters is needed to react completely (with nothing left over) with 0.500 L of 0.500 M Na2CO3 ?Express your answer to three significant figures and include the appropriate units.
Answers
ANSWER
The volume of the acid is 0.5 L
EXPLANATION
Given that;
The molarity of HCl is 1.00 M
The volume of Na2CO3 is 0.500 L
The molarity of Na2CO3 is 0.500M
To find the volume of HCl needed, follow the steps below
Step 1; Write the balanced equation for the reaction
[tex]\text{ 2HCl\lparen aq\rparen }+\text{ Na}_2CO_{3(aq)}\text{ }\rightarrow\text{ 2NaCl}_{(aq)}\text{ }+\text{ CO}_{2(g)}\text{ }+\text{ H}_2O_{(l)}[/tex]In the above reaction, 2 moles of HCl is needed to completely react with Na2CO3 .
Step 2; Apply the molarity concept to find the volume of the acid
[tex]\text{ }\frac{C_AV_A}{C_BV_B}\text{ = }\frac{n_A}{n_B}[/tex]Where
CA is the concentration of the acid
VA is the volume of acid
CB is the concentration of the base
VB is the volume of the base
nA is the number of moles of acid
nB is the number of moles of base
Step 3; Substitute the given data into the formula in step 2
[tex]\begin{gathered} \text{ }\frac{1\text{ }\times\text{ V}_A}{0.5\text{ }\times\text{ 0.5}}\text{ = }\frac{2}{1} \\ \text{ Cross multiply} \\ \text{ 1 }\times V_A\text{ = 2 }\times\text{ 0.5 }\times\text{ 0.5} \\ \text{ V}_A\text{ = 0.5 L} \end{gathered}[/tex]Hence, the volume of the acid is 0.5 L
Calculate the amount of heat needed to boil 41.1 g of water (H2O), beginning from a temperature of 84.7 C . Be sure your answer has a unit symbol and the correct number of significant digits.
Answers
Explanation:
We need to go through to stages to boil 41.1 g of water. We have to heat the sample of water from 84.7 °C to 100 °C (the boiling point) And then we have to provide enough heat to boil all the sample of water.
a) Heating from 84.7 °C to 100 °C:
This is calculated using the formula:
Q₁ = m * C * ΔT
Where Q₁ is the amount of heat, m is the mass of the sample, C is the specific heat of water and ΔT is the temperature change. We already know these values:
m = 41.1 g
C = 4.184 J/(g*°C)
ΔT = Tfinal - Tinitial = 100 °C - 84.7 °C
ΔT = 15.3 °C
Replacing these values we can get the amount of heat necessary for the first step:
Q₁ = m * C * ΔT
Q₁ = 41.1 g * 4.184 J/(g°C) * 15.3 °C
Q₁ = 2631 J
b) Boiling 41.1 g of water:
To find the amount of heat that we need to provide to the sample of water to completely boil it we can use this formula:
Q₂ = m * Cv
Where Cv is the latent heat of vaporization.
Cv = 2256 J/g
Q₂ = m * Cv
Q₂ = 41.1 g * 2256 J/g
Q₂ = 92721 J
c) Total amount of heat:
Qtotal = Q₁ + Q₂
Qtotal = 2631 J + 92721 J
Qtotal = 95352 J = 95400 J
Qtotal = 95.4 kJ
Answer: The amount of heat needed to boil the sample of water is 95.4 kJ or 95400 J.
can someone help me with this question
Answers
Explanation:
DataP = 1.3 atm
V = 2.1 L
T = 75 °C = 75 + 273 = 348 K
R = 0.0821 atm.L / mol.K
n = ?
SolutionAs, PV = nRT
therefore, n = PV/RT
n = 1.3 x 2.1 / 0.0821 x 348
n = 0.096 mole
Which part of an amino acid is different between the 20 different amino acids?• R-Group• Nitrogen Group• H-Group• COOH Group
Answers
The sides groups are what make each amino acid different from the others. Of the 20 side groups used to make proteins, there are two main groups: polar and non-polar. These names refer to the way the side groups, sometimes called "R" groups.
Using that,
In conclusion, the part of an amino acid that is different between the 20 different amino acids is the R-Group.
ANSWER:
• R-Group
C3H7OH + O2 -> CO2 + H20balance the equation then find mole ratio of oxygen to water, how many mores of carbon dioxide are produced when 4.6 mol of oxygen react, and how many molecules of C3H7OH will react with 4.6 L of O2?
Answers
mole ratio = 9:8
moles of CO2 = 3.07moles
molecules of C3H7OH = 2.75 * 10^22 molecules
ExplanationsThe balanced form of the chemical rreaction is as shown below;
[tex]2C_3H_7OH+9O_2\rightarrow6CO_2+8H_2O[/tex]The mole ratio of oxygen to water as shown in the equation is 9:8
Given the following parameter
mole of oxygen that reacted = 4.6moles
According to stoichiometry, 9moles of oxygen produces 6 moles of caron dioxide. The moles of CO2 that is required will be expressed as:
[tex]\begin{gathered} moles\text{ of CO}_2=\frac{6}{9}\times4.6moles\text{ of O}_2 \\ moles\text{ of CO}_2=3.07moles \end{gathered}[/tex]Hence the moles of CO2 that will be produced is 3.07moles
Also if 4.6L of O2 reacted, the moles of oxygen that reacted will be;
[tex]\begin{gathered} moles\text{ of O}_2=\frac{4.6}{22.4}(1mole\text{ = 22.4L}) \\ moles\text{ of O}_2=0.205moles \end{gathered}[/tex]According to stochiometry, 2 moles of C3H7OH reacts with 9moles of oxygen, the moles of C3H7OH required will be:
[tex]\begin{gathered} moles\text{ of C}_3H_7OH=\frac{2}{9}\times0.205 \\ moles\text{ of C}_3H_7OH=0.0456moles \end{gathered}[/tex]Convert the moles of C3H7OH to molecules as shown
[tex]\begin{gathered} molecules\text{ of }C_3H_7OH=0.0456\times6.02\times10^{23} \\ molecules\text{ of }C_3H_7OH=0.275\times10^{23} \\ molecules\text{ of }C_3H_7OH=2.75\times10^{22}molecules \end{gathered}[/tex]How many grams of H3PO4 could be produced from 94 g of H2O and 186 g of PCL5 according to the equation below?
Answers
Answer:
[tex]89.4\text{ g of H}_3PO_4[/tex]Explanation:
Here, we want to get the mass of H3PO4 produced
We start by getting the balanced equation of reaction:
[tex]PCl_5\text{ + 4H}_2O\text{ }\rightarrow\text{ 5HCl + H}_3PO_4[/tex]The mass of H3PO4 produced would be based on the limiting reactant
The limiting reactant here is the one that would produce less amount of the product H3PO4
Firstly, let us get the number of moles of H3PO4 produced by each of the reactants
To get this, we start by getting the number of moles of each that reacted
We get this by dividing the masses by the molar masses
For water, we have it that the molar mass is 18 g/mol
Thus, the number of moles would be:
[tex]\frac{94}{18}\text{ = 5.2 moles}[/tex]From the balanced equation of reaction:
4 moles of water produced 1 mole of H3PO4
5.2 moles of water will produce x moles of H3PO4
Thus:
[tex]\begin{gathered} x\text{ }\times4\text{ = 5.2}\times1 \\ x\text{ = }\frac{5.2}{4}\text{ = 1.3 mole} \end{gathered}[/tex]For PCl5
The molar mass is 208 g/mol
Thus,we have the number of moles as:
[tex]\frac{186}{208}\text{ = 0.894 mol}[/tex]From the equation of reaction:
1 mol of PCl5 produced 1 mol of H3PO4
That means 0.894 mol of PCl5 produced 0.894 mol of H3PO4
Since PCl5 produced a lesser amount of moles of water, that means, it is the limiting reactant
To get the mass of H3PO4 produced, we multiply this number of moles by
the molar mass of H3PO4
The molar mass of H3PO4 is 100 g/mol
Thus, we have the mass of H3PO4 produced as:
[tex]0.894\text{ }\times\text{ 100 = 89.4 g}[/tex]29.Which of the following is not an example of an acid-base indicator?Select one:a. Phenolphthaleinb. Litmus paperc. Hydrion paperd. Filter paper
Answers
Answer:
[tex]D\text{ : Filter Paper}[/tex]Explanation:
Here, we want to select the option that is not an acid-base indicator from the options
Acid-base indicators are colored materials that are expected to change color based on the pH
A filter paper is a material used in filtration
As such, it cannot be used as an indicator
31.The correct name for the compound that has the formula BaCl2 is...Select one:a. Baride chlorine.b. Barium chloride.c. Barium chlorine.d. Barium chlorite.
Answers
ANSWER
option B
EXPLANATION
Given that;
The molecular formula of the compound is given as BaCl2
The compound contain a metal and a non-metal
Ba means Barium
Cl2 is chloride because its a diatomic gas
Then BaCl2 is called Barium chloride
Therefore, the correct answer is option B
What is the chemical reaction for calcium
Answers
Answer:
I think the answer is calcium reacts with oxygen, forming a thin layer of CaO
Explanation:
I hope this helps
Maria is an 18 year old girl whose parathyroid has stopped functioning properly. What does this mean for the calcium levels in her
body?
Answers
Maria is an 18 year old girl whose parathyroid has stopped functioning properly, this mean calcium levels in her body decreased.
Calcium (Ca) is a chemical element and an alkaline-earth metal from Periodic Table Group 2 (IIa). It is the fifth most abundant element in the Earth's crust and the most prevalent metallic element in human tissue.
The ancients made considerable use of lime (calcium oxide, CaO), a calcium compound. Sir Humphry Davy first extracted the lightweight, silvery metal itself in 1808 after distilling mercury from an amalgam created by electrolyzing a combination of lime and mercuric oxide. The Latin word for lime, calx, served as the inspiration for the element's name. The cosmic abundance of calcium is thought to be 4.9 104 atoms, and it makes up 3.64 percent of the crust of the Earth and 8 percent of the crust of the Moon.
To know more about calcium visit : https://brainly.com/question/3569638
#SPJ9
Can you help me answer 13 and explain the answer
Answers
Assuming that this question is comparing two situations, only water as situation 1 and water + calcium chloride as situation 2. In this case, we will have the salt interacting with water in something called Colligative properties, which depending on the concentration of the solute, it will cause changes in physical state change of the solution. Salts in water tend to cause two things, regarding physical change, they raise the boiling point and lower the freezing point. Which is the case for this question, we have a solution with salt + water, therefore we will have a higher boiling point and a lower freezing point, number 3
2.Solid iron combines with oxygen gas to form solid iron(III) oxide. Which of the following equations best describes this reaction?Select one:a. Ab. Bc. Cd. D
Answers
ANSWER
option B
EXPLANATION;
The chemical symbol of iron is Fe
The chemical symbol of oxygen is O2
[tex]\text{ 4Fe + 3O}_2\text{ }\rightarrow\text{ 2Fe}_2O_3[/tex]Therefore, the correct answer is B
Need it quickly ples
Answers
Based on the model , the statement best compares the amounts energy in the reactants and the products of photosynthesis is since making sugar uses energy, the combined energy in the bond CO₂ and H₂O is greater than that in the bonds of O₂ and C₆H₁₂O₆
The plants make sugar using the energy that comes from sunlight and it will convert into the carbon dioxide and the water. this is called as photosynthesis. this process occurs in the chloroplast in the plant cell.
Thus, Based on the model , the statement best compares the amounts energy in the reactants and the products of photosynthesis is since making sugar uses energy, the combined energy in the bond CO₂ and H₂O is greater than that in the bonds of O₂ and C₆H₁₂O₆
To learn more about photosynthesis here
https://brainly.com/question/1757345
#SPJ1
I need help with my homework I need help with number 3Given: 1 foot is equivalent to 12 inches Given: 1 yard is equivalent to 3 feet Given: 1 mile is equivalent to 5, 280 feet The number of yards in 0.45 miles is
Answers
Answer:
The number of yards is 792.
Explanation:
To convert 0.45 miles to yards it is necessary to use the equivalence from mile to feet, and then the equivalence from feet to yard:
[tex]0.45\text{miles}\cdot(\frac{5,280feet}{1\text{mile}})\cdot(\frac{1\text{yard}}{3\text{feet}})=792\text{yards}[/tex]So, it is 792 yards.
Oxygen gas is made up of diatomic non-polar covalent molecules. This leads to very weak attraction between molecules. Which statement would this best support?
Answers
The oxygen gas is non polar because there is no interaction between the molecules of the gas.
What is a diatomic molecule?A diatomic molecule is one that has only two atoms in it. These atoms are usually atoms of the same element in the majority of the cases that we encounter. Given the fact that the both atoms belong to the same substance, the electronegativity difference between the atoms is zero.
Given that the electronegativity difference between the atoms is zero, there is little or no interaction between the molecules of the substance. Thus the very weak intermolecular interaction is coming from the fact that there is no kind of interaction between the molecules.
Thus oxygen gas is covalent and the molecules do not interact with each other because the bond is nonpolar.
Learn more about diatomic molecules:https://brainly.com/question/11815815
#SPJ1
Write All Gas Laws Meaning And Example Situation.
Answers
Gas laws discover the relationship of pressure, temperature, volume and amount of gas.
1. Boyles Law
Boyle's Law tells us that the volume of gas increases as the pressure decreases, meaning pressure is inversely propotional to volume.
Equation used for Boyles Law: P1V1 = P2V2
Example: A 17.50mL sample of gas is at 4.500 atm. What will be the volume if the pressure becomes 1.500 atm, with a fixed amount of gas and temperature?
So in this example we are given, P1 and P2, we are also given V1 and we want to know V2. What will happen to the volume if the pressure is increased.
V2 = P1V1/P2
V2 = (4.500 atm x 17.50 mL)/1.500atm
V2 = 52.50mL
This example proves that when the pressure is increased, the volume also increases.
2. Charles Law
Charles' Law tells us that the volume of gas increases as the temperature increases. Volume is directly proportional to Temperature.
The equation used: V1/T1 = V2/T2
Example: A sample of Carbon dioxide in a pump has volume of 20.5 mL and it is at 40.0 oC (=313.15 K). When the amount of gas and pressure remain constant, find the new volume of Carbon dioxide in the pump if temperature is increased to 65.0 oC (=333.15 K).
V2 = (V1 x T2)/T1
V2 = (20.5 mL x 333.15 K)/313.15K
V2 = 21.8 mL
The volume increased as the temperature increased.
3. Avogadro's Law tell us that the volume of gas increases as the amount of gas increases. Volume(V) is directly proportional to the Amount of gas(n).
Equation: P1/n1 = P2/n2 and V1/n1 = V2/n2
Example: A 3.80 g of oxygen gas in a pump has volume of 150 mL. constant temperature and pressure. If 1.20g of oxygen gas is added into the pump. What will be the new volume of oxygen gas in the pump if temperature and pressure held constant?
This problem is asking for V2 of oxygen. We have to first calculate the number of moles of Oxygen.
n1 = 3.80/(15.999 x 2)
n1 = 0.1188 mol
n2 = 1.20/(15.999 X 2)
n2 = 0.0375 mol
V2 = (V1 x n2)/n1
V2 = (150.0mL x 0.0375)/0.1188
V2 = 47.3485 mL
4. The ideal gas law
The ideal gas law is the combination of the three simple gas laws.
Equation: pV = nRT
Example: At 655mm Hg and 25.0oC, a sample of Chlorine gas has volume of 750mL. How many moles of Chlorine gas at this condition?
So we want number of moles.
n = PV/RT
n = 655mmHg x( 1 atm/760 mmHg) x 0.75 L/0.082057L⋅atm⋅mol-1.K-1 x 298.15
n = 0.026 mol
Where do the following boxes on the right belong to?
Answers
According to the question, we are to categorize the definitions in the box as elements, compounds, or mixtures.
For ELEMENTS
• Since element,s consist of only one kind of atom,, hence they are ,substances made up of one type of particle (atoms or molecules)
• Since matter is anything that has mass and occupies space and is made up of substances called elements, hence, they are anything that has mass and volume
,• Cannot be broken into simpler kinds, of matter since elements are made of atoms.
For COMPOUNDS
• Compounds can only be separated by chemical means but not by physical means. Hence we can say that ,compounds cannot be separated by physical means, but can be chemically
• Consists of atoms of two or more different elements bonded together
,• Has properties unique to the element that makes it up
FOR MIXTURES
• Since the mixture consists of, two or more different elements ,and/or compounds physically, hence they can be separated by physical means.
• They can be separated into simpler types of matter by physical means and keeps the, properties of parts, that make it up.
Calculate the % composition of Al2O3?Group of answer choices47.1% AL, 52.9% O52.9% Al, 47.1% O28.3% Al, 71.7% O71.1% Al, 28>3% O62.8% Al, 37.2% O
Answers
First, we need to find the values of molar mass of Al and O at the periodic table:
Al : 27 g/mol
O: 16 g/mol
Now let's calculate the molar mass of Al2O3:
(2x27) + (16x3)
54 + 48 = 102 g/mol
Now let's calculate the percent of each element:
For Al:
102 ---- 100%
54 ---- x%
x = 52.9%
For O:
102 ---- 100%
48 ---- y%
y = 47.1%
Answer: 52.9% Al, 47.1% O
A jug of vinegar is 15% (by mass) acetic acid. If a container holds 500 g of this product, how much acetic acid does it contain?
Answers
Answer:
It contains 75g of acetic acid.
Explanation:
With the percent mass (15%) and the mass of the solution of vinegar, we can calculate the amount of acetic acid, using the percent by mass formula:
[tex]\begin{gathered} PercentByMass=\frac{MassOfSolute}{MassOfSolution}*100\% \\ 15\%=\frac{MassOfSolute}{500g}\times100\operatorname{\%} \\ \frac{15\%}{100\operatorname{\%}}*500g=MassOfSolute \\ 75g=MassOfSolute \\ \end{gathered}[/tex]So, it contains 75g of acetic acid.