Answers
Answer:
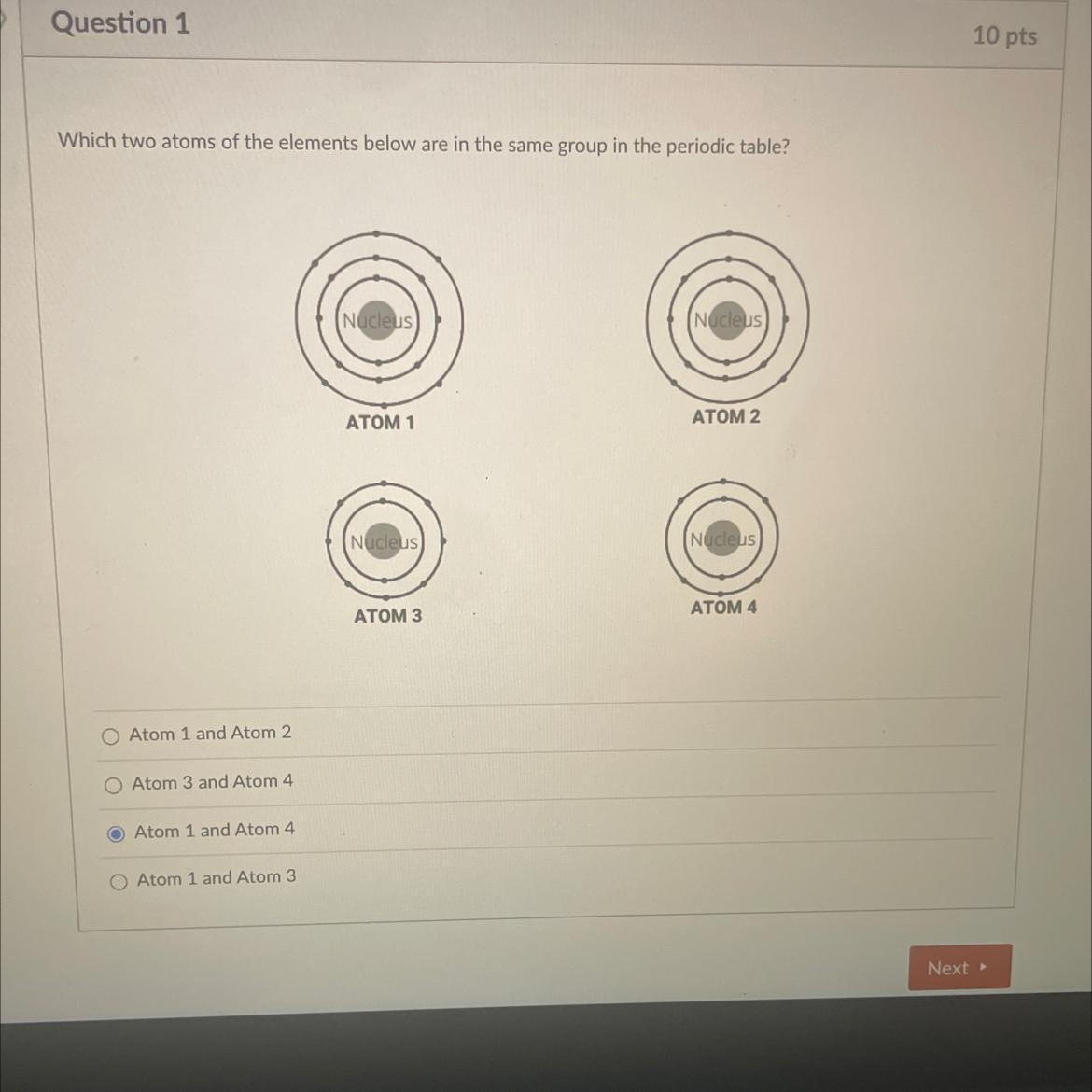
Atom 1 and Atom 4 of the elements below are in the same group in the periodic table.
Related Questions
Select the structure that correspondsto the name:pentanoic acidA. CH3CH₂CH₂CH₂COOHB.C. bothCOOH
Answers
Answer
C. both
Explanation
Structures in option A and option B both corresponds to the name: pentanoic acid.
The correct answer is C. both
Predict the formula for each ionic compoundbarium sulfate:lithium nitrate :cesium sulfide:
Answers
INFORMATION:
We have the following ionic compounds:
- barium sulfate
- lithium nitrate
- cesium sulfide
And we must write their formulas
STEP BY STEP EXPLANATION:
1. Barium sulfate:
To write its formula, we need to use that:
- the element symbol for Barium is Ba, and it has a charge of 2+
- in the given table, sulfate is a polyatomic ion represented by SO4, and it has a charge of 2-
Now, we must add up the charges to verify if the ionic compound is neutral
[tex]2^++2^-=0[/tex]Since the compound has a net charge of zero, we can write its formula as
[tex]BaSO_4[/tex]2. Lithium nitrate:
To write its formula, we need to use that:
- the element symbol for Lithium is Li, and it has a charge of 1+
- in the given table, nitrate is a polyatomic ion represented by NO3, and it has a charge of 1-
Now, we must add up the charges to verify if the ionic compound is neutral
[tex]1^++1^-=0[/tex]Since the compound has a net charge of zero, we can write its formula as
[tex]LiNO_3[/tex]3. Cesium sulfide:
To write its formula, we need to use that:
- the element symbol for Cesium is Cs, and it has a charge of 1+
- the element symbol for sulfide is S, and it has a charge of 2-
Now, we must add up the charges to verify if the ionic compound is neutral
[tex]1^++2^-=1^-[/tex]Since the compound has a net different from zero, we need to analyze the compound.
So, if we have just one atom of Cs, the charge would be 1+, but if we take two atoms the charge would be 2(1+) = 2+
Now, verifying the net charge
[tex]2^++2^-=0[/tex]Now, we can write its formula as
[tex]Cs_2S[/tex]ANSWER:
barium sulfate:
[tex]BaSO_4[/tex]lithium nitrate :
[tex]LiNO_3[/tex]cesium sulfide:
[tex]Cs_2S[/tex]This is my question in imageIf 16.0 grams of aluminum oxide were actually produced, what is the percent yield of the reaction below given that you start with 10.0 g of Al and 19.0 grams of O2?Reaction: 4Al + 3O2 → 2Al2O3Group of answer choices70%39.6%75.0%100%85.0%
Answers
Answer: the percent yield of this reaction was 84.7% and the best option to answer the question is the last one (letter E, 85.00%)
Explanation:
The question requires us to determine the percent yield of a reaction, given the amount of product obtained, the chemical equation and the amount of reactants used.
The following information was provided by the question:
mass of Al2O3 produced = 16.0 g
mass of Al used = 10.0 g
mass of O2 used = 19.0 g
balanced chemical equation:
[tex]4Al+3O_2\rightarrow2Al_2O_3[/tex]To solve this problem, we'll need to go through the following steps:
1) Calculate the number of moles used of each reactant;
2) determine the limiting reactant from the stoichiometry of the reaction and the amount of reactants used;
3) calculate the theoretical yield of the reaction, or, in other words, the amount of Al that should be produced, considering the limiting reactant;
4) calculate the percent yield of the reaction.
Next, we'll go through these steps to solve the problem:
1) Calculating the number of moles of each reactant
We can use the following equation to determine the amount of moles of Al and O2 that were used in the reaction:
[tex]n=\frac{m}{MM}[/tex]where n is the number of moles (in mol), m is the mass of the sample (in grams) and MM is the molar mass of the compound (in g/mol).
Knowing that the molar masses of Al and O2 are 26.98 and 31.98 g/mol, respectively, we can calculate the number of moles of each reactant as:
[tex]\begin{gathered} n_{Al}=\frac{10.0g}{26.98g/mol}=0.371mol\text{ Al} \\ n_{O_2}=\frac{19.0g}{31.98g/mol}=0.594mol\text{ }O_2 \end{gathered}[/tex]
Therefore, 0.371 and 0.594 moles of Al and O2 were used in the reaction, respectively.
2) Determining the limiting reactant.
From the balanced chemical equation, we can see that 4 moles of Al are necessary to react with 3 moles of O2. Thus, we can determine how many moles of O2 would be necessary to react with 0.371 moles of Al:
4 mol Al ------------------- 3 mol O2
0.371 mol Al ------------- x
Solving for x, we'll have:
[tex]x=\frac{(3mol\text{ }O_2)\times0.371mol\text{ Al\rparen}}{(4mol\text{ Al\rparen}}=0.278mol\text{ }O_2[/tex]Therefore, 0.278 moles of O2 would be necessary to react with the used amount of Al (0.371 mol). Since the actual amount of O2 used is greater than the necessary amount, we can say that O2 is the excess reactant and Al is the limiting reactant.
3) Calculating the theoretical amount of Al2O3 produced
Now that we know that Al was the limiting reactant in this reaction, we can determine how much Al2O3 should be produced in the reaction.
From the balanced chemical equation, we can see that 4 moles of Al are necessary to produce 2 moles of Al2O3. Thus, we can write:
4 mol Al --------------------- 2 mol Al2O3
0.371 mol Al --------------- y
Solving for y, we'll have:
[tex]y=\frac{(2mol\text{ A}l_2O_3)\times(0.371mol\text{ Al\rparen}}{(4mol\text{ Al\rparen}}=0.186mol\text{ A}l_2O_3[/tex]Therefore, with the amount of Al used, 0.186 moles of Al2O3 would be produced.
We can convert this amount in mass of Al2O3 using its molar mass (MM = 101.96 g/mol):
[tex]\begin{gathered} n=\frac{m}{MM}\rightarrow m=n\times MM \\ n_{Al_2O_3}=(0.186mol)\times(101.96g/mol)=18.91g \end{gathered}[/tex]Therefore, 18.9 g of Al2O3 should be obtained from the given mass of Al given.
4) Calculating the percent yield of the reaction
Note that the amount of Al2O3 expected, from the amount of reactants given, was 18.9g, but only 16.0g of the product was obtained. We can calculate the percent yield of a reaction using the following equation:
[tex]\begin{gathered} percent\text{ yield = }\frac{actual\text{ yield \lparen g\rparen}}{theoretical\text{ yield \lparen g\rparen}}\times100\% \\ \\ \%yield=\frac{16.0g}{18.9g}\times100\%=84.7\% \end{gathered}[/tex]Therefore, the percent yield of this reaction was 84.7% and the best option to answer the question is the last one (letter E, 85.00%).
Finish the following equations and list whether they will occur need one product to be insoluble. CuSO4 + Na2CO3 -->
Answers
The reaction will occur.
[tex]CuSO_{4}+Na_{2}CO_{3}\operatorname{\rightarrow}Na_{2}SO_{4}+CuCO_{3}[/tex]Explanations:The given chemical reaction is a double displacement reaction. In this reaction, the compoiunds will react to produce two different compounds.
The balanced form of the reaction is expressed as:
[tex]CuSO_4+Na_2CO_3\rightarrow Na_2SO_4+CuCO_3[/tex]You can see that the reaction of cupper(II)sulphate and sodium carbonate produces sodium sulphate and copper(II)carbonate
Since most carbonate compounds are insoluble, hence the reaction will occur
For a 4s orbital ,what are the possible values of n, l and ml.
Answers
In order to know the values of the quantum numbers n, l and ml for al s4 orbital we go to a quantum table:
And as we can see to de 4s orbital correspond only the values:
n=4
l=0
ml=0
Naming compoundsPb3(AsO4)4
Answers
ANSWER
Lead (IV) Arsenate
STEP-BY-STEP EXPLANATION:
The compound consists of two elements which are; Lead and Arsenic, and oxygen
Combination off oxygen and Arsenic gives Arsenate
The name of the compound is Lead (VI) Arsenate
How many grams of FeSO4 (molar mass = 151.9 g/mol) are present in a 200.0 mL sample of a 0.250 M solution? Enter your answer with 3 significant figures and do not include the unit
Answers
7.60grams
Explanations:The formula for calculating the molarity of a solution is expressed as;
[tex]molarity=\frac{mole}{volume}[/tex]Given the following parameters
molarity of solution = 0.250M
volume of sample = 200mL
Substitute to determine the mole of the solute
[tex]\begin{gathered} mole\text{ of }FeSO_4=molarity\times volume \\ mole\text{ of }FeSO_4=\frac{0.250mol}{L}\times0.2L \\ mole\text{ of }FeSO_4=0.05moles \end{gathered}[/tex]Determine the required mass of FesO4
[tex]\begin{gathered} Mass\text{ of FeSO}_4=mole\times molar\text{ mass} \\ Mass\text{ of FeSO}_4=0.05\times151.9 \\ Mass\text{ of FeSO}_4=7.595grams \\ Mass\text{ of FeSO}_4=7.60grams \end{gathered}[/tex]Hence the mass of FeSO4 is 7.60grams
Give an example of heat energy out vs. heat energy in during chemical reaction
Answers
An exothermic reaction is a reaction that releases energy. One example of this type of reaction is burning gas in a gas oven.
An endothermic reaction is a reaction that absorbs energy. One example of this type of reaction is
Which solution has the highest boiling point?A. 0.1 m CH4N₂OB. 0.06 m HCIC. 0.05 m CaCl2D. 0.04 m (NH4)3PO4E. 0.07 m NaCl
Answers
Answer
D. 0.04 m (NH4)3PO4
Explanation
Boiling point elevation depends on the solvent, of course, but we're assuming water, and upon the concentration of the solute and how many particles into which the solute breaks. Colligative properties like boiling point elevation, depend on the total number of particles in solution. The solvent used here is water. So elevation in boiling point will be above a boiling point of water for all solutions.
ΔT= i x Kb x m,
Where i = van Hoff's factor, Kb = boiling point elevation constant of water = 0.512, m = molality
Higher ΔT will have a higher boiling point.
For 0.1 m CH4N₂O,
i = 1
ΔT = 1 x 0.512 x 0.1 = 0.0512
For 0.06 m HCI;
i = 2
ΔT = 2 x 0.512 x 0.06 = 0.06144
For 0.05 m CaCl2
i = 3
ΔT = 3 x 0.512 x 0.05 = 0.0768
For 0.04 m (NH4)3PO4;
i = 4
ΔT = 4 x 0.512 x 0.04 = 0.08192
For 0.07 m NaCl
i = 2
ΔT = 2 x 0.512 x 0.07 = 0.07168
0.04 m (NH4)3PO4 has the highest ΔT.
Therefore, the solution that has the highest boiling point would be D. 0.04 m (NH4)3PO4.
) What is the most notable difference between particles in the solid phase and the liquid phase?
Answers
Answer: Particles in the solid phase are tightly compact and they vibrate, while particles in the liquid phase move freely, randomly, and with more room to move
Explanation:
.calculate amount of heat required to rais the temperature of 50 g og copper by 70 K? (specific heat of copper is 0.385 J/g.K.
Answers
To determine the heat required (Q) to increase the temperature of a substance we can apply the following equation:
[tex]Q=mCp\Delta T[/tex]where,
m is the mass of the substance, 50g
Cp is the specific heat of the substance, 0.386J/g.K
deltaT is the temperature difference, 70K
Now, we have the values we need to calculate the heat required, we replace the known data into the equation:
[tex]Q=50g\times0.385\frac{J}{g.K}\times70K=1347.5J[/tex]The heat required to raise the temperature of 50 g of copper by 70 K will be 1347.5J
Calculate the mass of 124.50 cm³ of titanium (density = 4.540 g/cm³).
Give your answer to four significant figures
Mass=____g
Answers
Mass of titanium having density 4.540 g/cm³) is 565.2g
What are significant figures?
Significant figures are those digits in a number that are meaningful in terms of precision and accuracy. The larger the number of significant figures obtained in a measurement, greater is the accuracy of the measurement and vice-versaRules for calculating significant figures:
When adding or subtracting, round the answer to the least number of decimal placesex: 1.457 + 83.2 = 84.657 , rounds to 84.7
When multiplying or dividing , round the answer to the least number of significant figuresex: 4.36 * 0.00013 = 0.0005668 , round to 0.00057
here, density = mass/ volume
mass = density * volume
= 4.540 * 124.50
= 565.23
Value of density has 4 significant figures and value of volume has 5 significant figures. so we choose density, since it has least significant figures and round to 4 digits.
We get, 565.2g as the mass of titanium
Learn more about significant figures at https://brainly.com/question/24491627
#SPJ13
List the following aqueous solutions in order of increasing melting point. (The lastthree are all assumed to dissociate completely into ions in water.)(a) 0.1 m sugar(b) 0.1 m NaCl(c) 0.08 m CaCl2(d) 0.04 m Na2SO4
Answers
0.1M sugar < 0.1 M NaCl < 0.08M CaCl2 < 0.04 M Na2SO4
Explanations:First we must understand that the higher thecnoncentration of a compound, the lower the melting point. This shows that 0.04 m Na2SO4 will have highest melting point followed by 0.08 M CaCl2
Since 0.1M of sBgar and 0.1M of NaCl have the same molarity, we will determine the effect of solute and ionic bonding between compound on melting point. Since common salt (NaCl) is made up of stronger ionic bonds, it will have higher melting point than sugar.
The increasing order of their melting point wile be 0.1M sugar < 0.1 M NaCl < 0.08M CaCl2 < 0.04 M Na2SO4
Air is not a compound because it(A) has variable composition(B) has a stoichiometry formula(C)can be purified(D) has a fixed compositiona
Answers
Answer
(A) has variable composition
Explanation
Air is a mixture, but not a compound because it can be separated into different components such as oxygen, nitrogen, etc through the process of fractional distillation.
Therefore, air is not a compound because it has a variable composition.
The correct answer is (A) has variable composition
Arrange in order of increasing ionization energy. (Use the appropriate <, =, or > symbol to separate substances in the list.)
Answers
Explanation:
The 3 elements are As, Ga, and Se.
Please, look at the next picture:
According to the 2 pictures above, the right arrangement will be:
Ga < As < Se
Ga has the lowest ionization energy and Se has the highest ionization energy.
Answer: Ga < As < Se
You are given a bulb, one metre copper wire, a switch and a battery. How could you use them to distinguish the samples of metals and non-metals? Write the usefulness of this test in distinguishing metals and non-metals.
Answers
With the given materials following experiment can be done
Electrical conductivity test:
Metals are good conductors of electricity, therefore when you join metals with a battery, wire, and bulb, you get a bulb.
Similarly, if nonmetals are poor electrical conductors, they may not ignite the bulb when attached to a wire and a battery.
Generally, the above method can be used to identify metals and non-metals. But there are some exceptions also,. Graphite, an allotrope of non-metal carbon, is a good conductor of electricity.
Learn more about Metals and their properties;
https://brainly.com/question/25090336
The periodic chart has many different types of elements. Some elements are metals, some are nonmetals, and yet others are metalloids. Therefore, by electrical conductivity test, we can detect metal and non metal.
What is metal ?Metals are hard, conduct electricity, are ductile, lustrous, and malleable materials.
Since metals have free electrons that's why they can conduct electricity. Since atoms in metals are very closely packed in a definite crystal solid. It is not brittle that is it can not be broken down easily.
Metals carry electricity well, therefore when you combine metals with a battery, wire, and bulb, you get a light. Similarly, if nonmetals are poor electrical conductors, when connected to a wire and a battery, they may not light the bulb.
Therefore, by electrical conductivity test, we can detect metal and non metal.
Learn more about the metals, here:
https://brainly.com/question/27859211
#SPJ2
With all the steps please:A solution is prepared by dissolving 166.0 g of potassium iodide, Kl, in water to a total volume of 1,000 mL. Calculate the molarity of this solution.
Answers
Explanation:
Molarity, or concentration in amount of matter (mol/L), is the ratio between the amount of matter in the solute (n) and the volume of the solution in liters (V): M = n/V
Here we have:
m = 166 g of KI (solute)
V = 1000 mL = 1L (solution)
So we need to transform 166 g of KI into moles. We use the following formula:
n = m/MM
MM of KI is 166 g/mol
n = 166/166
n = 1 mol
So:
M = 1/1
M = 1 mol/L
Answer: M = 1 mol/L
An aqueous solution of a nonionic (that is, nonelectrolyte) solute has a freezing point of -0.435 °C. The solution contains 2.49 g solute dissolved in 95.0 g H2O. What is the molar mass of the solute? g/mol
Answers
To answer this question, we have to use the formula of the cryoscopic descent:
[tex]\Delta T_c=k_f\cdot m[/tex]Where ΔTc is the difference between the freezing points of the pure solvent and the solution, kf is the cryoscopic constant (that for water it has a value of 1.86°C*kg/mol and m is the molality of the solution.
The first step we have to follow is to find the molality of the solution using this formula and the given values.
Estimate the difference between the freezing point of the water and the one of the solution:
[tex]\Delta T_c=0\degree C-(-0.435\degree C)=0.435\degree C[/tex][tex]\begin{gathered} 0.435\degree C=1.86\degree Ckg/mol\cdot m \\ m=\frac{0.435\degree C}{1.86\degree Ckg/mol} \\ m=0.234mol/kg \end{gathered}[/tex]The molality of the solution is 0.234mol/kg.
Now we have to use this molality to find the amount of moles present in the solution.
Molality equals moles of solute/kg of solvent. We know that there are 95.0g of solvent, by converting this mass to kg and using the molality we can find the amount of moles of solute present in the solution, this way:
[tex]95.0g\cdot\frac{1kg}{1000g}=0.095kg[/tex][tex]0.095kg\cdot\frac{0.234mol}{kg}=0.02223mol[/tex]It means that there are 0.02223 moles of solute in the solution.
Our last step to answer this question is to divide the mass of solute present in the solution by the amount of moles of it to find its molar mass:
[tex]\frac{2.49g}{0.02223mol}=112.01g/mol[/tex]It means that the molar mass of the solute is 112.01g/mol.
Explain the role electronegativity plays in the polarity of a molecule.
Answers
Electronegativity is the force of an atom to attract electrons to itself, and Polarity represents the distribution of electron density of a molecule.
When the atoms of a molecule have very different electronegativities, zones with different electron densities are generated, and poles are formed.
So, the role that electronegativity plays in the polariry of a molecule is very important because knowing the electronegativity of atoms we can know if a molecule is polar or nonpolar.
The taste of sour milk is lactic acid. What is the molecular formula for lactic acid if the percent composition is 40.00% C, 6.71% H, 53.29% O, and the approximate molecular mass is 90 amu?
Answers
Given the following parameters
Carbon = 40.0%
Hydrogen = 6.71%
Oxygen = 53.29%
Convert to mass
Carbon = 40.0grams
Hydrogen = 6.71grams
Oxygen = 53.29grams
Convert the mass to moles
Mole of carbon = 40.0/12 = 3.33moles
Mole of hydrogen = 6.71/1 = 6.71 moles
Mole of oxygen = 53.29/16 = 3.33moles
Divide by the lowest number of moles
Carbon: 3.33/3.33 = 1
Hydrogen: 6.71/3.33 = 2.01
Oxygen: 3.33/3.33 = 1
Determine the empirical formula
Empirical formula = CH2O
Determine the molecular formula
[tex]\begin{gathered} (CH_2O)_n=90 \\ (12+2(1)+16)n=90 \\ 30n=90 \\ n=\frac{90}{30} \\ n=3 \end{gathered}[/tex][tex](CH_2O)_3=C_3H_6O_3[/tex]Hence the molecular mass of the compound is C3H6O3
What amount (moles) is represented by each of these samples?b. 10.0 mg NO2c. 1.5 3 1016 molecules of BF3
Answers
To find the amount represented by 1.5x10^16 molecules of BF3 we have to use Avogadro's Number. It was stated that 1 mol of any substance contains 6.022x10^3 atoms or molecules of that substance, that is what we know as Avogadro's Number:
[tex]1.5\times10^{16}molecules\cdot\frac{1mol}{6.022\times10^{23}molecules}=2.5\times10^{-8}mol[/tex]The answer is 2.5x10^-8 moles of BF3.
Lead is listed below zinc in the activity series.
What will most likely happen when zinc particles are placed in lead nitrate solution? (1 point)
Zinc lead will form.
Oxygen will evolve.
No reaction will occur.
Zinc nitrate will form.
Answers
zinc nitrate and solid lead most likely happen when zinc particles are placed in lead nitrate solution
[tex]Pb(NO_3)_2aq.+Zn(s)= Zn(NO_3)_2(aq.)+Pb(s)[/tex]
Without the chemical element zinc (Zn), which is one of the most extensively used metals and is a member of Group 12 (IIb, or the zinc group) of the periodic table, life would not be possible. Zinc has important business implications.
The average amount of zinc in the Earth's crust per tonne is 65 grams (2.3 ounces), which is a little more than copper. Nearly all of the world's zinc ore is composed of the principal zinc mineral sphalerite and its oxidation byproducts, smithsonite and hemimorphite. Australia, New Zealand, and the US are said to have discovered native zinc resources. China, Australia, and Peru are the top three producers of zinc in the beginning of the twenty-first century.
To know more about zinc visit: https://brainly.com/question/24711517
#SPJ1
Which of the following is a measure of the amount of space that an object occupies?MassVolumeDensityWeight
Answers
Step 1 - Understanding the definition of each term
Mass: it measures the amount of matter of a body
Volume: it measures the space occupied by a body
Density: it's the mass divided by the volume
Weight: it is an indirect measure of the mass, since it also includes the action of gravity
Step 2 - Answering the exercise
As we can see, the definition that fits what is being asked is volume.
Answer: volume
Find the oxidation numbersd) Oxidation number of K in K2SO3e) Oxidation number of S in K2SO3f) Oxidation number of O in K2SO3g) Oxidation number of S in S8
Answers
Answer:
d) The oxidation number of K in K2SO3 is +1.
e) The oxidation number of S in K2SO3 is +4.
f) The oxidation number of O in K2SO3 is -2.
g) The oxidation number of S in S8 is 0.
Explanation:
In each case, we can use the Periodic Table of Elements to consult the oxidation number of the elements.
d) In this case, K is in the group 1 of the Periodic Table, so it has always an oxidation number of +1.
f) Oxygen (O) always has an oxidation number of -2 (except in peroxides which has -1).
e) Knowing that the molecule of K2SO3 is neutral (the total charge of the molecule is equal 0), we can sum all the charges of the elements in the molecule, to find out which oxidation number has S in this molecule:
[tex]\begin{gathered} K_2SO_3 \\ K\text{ charge: }(+1)*2=+2 \\ O\text{ charge: }(-2)*3=-6 \\ \end{gathered}[/tex]Now we have to sum them and find out the oxidation number of S that will made the total charge of the molecule equal 0:
[tex]\begin{gathered} K_2SO_3 \\ +2\text{ + S oxidation number -6 =0} \\ -4\text{ + S oxidation number =0} \\ -4+4=0 \end{gathered}[/tex]So, the oxidation number of S is +4.
g) Finally, in this case, the oxidation number of S in S8 is 0, because it is a pure compound.
Allen wakes up one morning to find that all of the cortical (compact) bone in his body has been replaced by trabecular (spongy)
bone. What changes will there be in the density, strength, and flexibility of his bones?
Answers
Its thickness, hardness, and flexibility of the bones will alter, becoming less dense, stronger, and more flexible.
What type of bone is trabecular bone?Trabecular or bone graft refers to the porous, spongy-appearing interior of bones, particularly vertebra and the ends of long bones. Compared to cortical bone, it has a larger surface area and undergoes more active remodeling.
Why is trabecular bone important?As the primary load-bearing bone in vertebral column and the bone that transfers stress from the joints to the cancellous bone in long bones, trabecular bone's elastic behavior must be studied. Additionally, it impacts the bone structure's strength and the fracture risk.
To know more about trabecular bone visit:
brainly.com/question/12977471
#SPJ1
Need help with chemistry 52.3 g of metal at a temperature of 126.2°C are placed in 71.2 g of water which abs an into Al temperature of 24.5°C. The final temperature of the metal and water is 35.5°C. What is the specific heat of the metal in units of J/g°C? Use 4.184 J/g°C for this problem?
Answers
Answer:
The specific heat of the metal is 0.69 J/g°C.
Explanation:
The given information from the exercise is:
• Metal,:
- Mass (massmetal): 52.3g
- Initial temperature (Tinitialmetal): 126.2°C
- Final temperature (Tfinalmetal): 35.5°C
• Water,:
- Mass (masswater):71.2g
- Initial temperature (Tinitialwater): 24.5°C
- Final temperature (Tfinalwater): 35.5°C
1st) It is necesary to write the Heat formula for both materials, the metal and water. In the case of water, we can solve the formula, because we have all the nedeed information:
• Metal,:
[tex]\begin{gathered} Q_m=m_{metal}*c_{metal}*(T_{fmetal}-T_{imetal}) \\ Q_m=52.3g*c_{metal}*(35.5_\degree C-126.2\degree C) \\ Q_m=-4,743.61g\degree C*c_{metal} \\ \end{gathered}[/tex]Water:
[tex]\begin{gathered} Q_w=m_{water}*c_{water}*(T_{fwater}-T_{\imaginaryI water}) \\ Q_w=71.2g*4.184\frac{J}{g\degree C}*(35.5\degree C-24.5\degree C) \\ Q_w=3,276.9J \end{gathered}[/tex]So, the heat absorbed by the water is 3,276.9 J.
2nd) In the end both materials reach the equilibrium temperature, because the heat released by the piece of metal is absorbed by the water, this is represented as:
[tex]-Q_m=Q_w[/tex]Finally, we have to replace the heat of the metal equation (Qm) and the heat of water (Qw) to calculate the specific heat of the metal:
[tex]\begin{gathered} -Q_{m}=Q_{w} \\ -(-4,743.61g)\degree C*c_{metal}=3,276.9J \\ 4,743.61g\degree C*c_{metal}=3,276.9J \\ c_{metal}=\frac{3,276.9J}{4,743.61g\degree C} \\ c_{metal}=0.69\frac{J}{g\degree C} \end{gathered}[/tex]So, the specific heat of the metal is 0.69 J/g°C.
What is the oxidation state of nitrogen in Mg(NO₂)₂?
Answers
The oxidation state of nitrogen in Mg(NO₂)₂ is +3.
An oxidation state is an indicator of how electronegative an atom is. The higher the oxidation state, the more electronegative the atom.
To find the oxidation state of Mg(NO₂)₂, first, we need to split the compound and the compound should be again split into elements. On splitting, we get, Mg and (NO₂). We need to sum up the oxidation states of each element so that we get the total oxidation state of the given compound
The oxidation state of Magnesium = +2
The oxidation state of Nitrogen = +3
The oxidation state of Oxygen = -2
Then the total oxidation state of Mg(NO₂)₂
Total oxidation state = + 2 + 3 - 2
Oxidation state of Nitrogen Mg(NO₂)₂ = +3
Therefore the oxidation state of Mg(NO₂)₂ is +3.
Thus in this way oxidation state of any compound could be found easily.
To know more about redox reactions, click below:
https://brainly.com/question/21851295
#SPJ9
Calculate the molarity of a solution made by adding 150.0 mL of water to 85.00 mL of a 0.157 M solution
Answers
Answer:
0.089 M
Explanation:
M1V1=M2V2
Total volume = 150 ml + 85 ml = 235ml
0.157(.085 L) = M2(.235L)
M2 = 0.05678723404 or 0.057 M
4. __H2SO4 + __Cr(OH)3 --> __Cr2(SO4)3 + __H2OYou have 4 moles of H2SO4. How many moles of H2O are produced?
Answers
The number of moles of H2O produced when 4 moles of H2SO4 are there is 8 moles of H2O
The reaction is __H2SO4 + __Cr(OH)3 --> __Cr2(SO4)3 + __H2O. The reaction is as follows;
2Cr(OH)3 + 3H2SO4 → Cr2(SO4)3 + 6H2O
3 moles of H2SO4 produces 6 moles of H2O
1 mole of H2SO4 produces 2 moles of H2O
4 moles of H2SO4 produces 8 moles of H2O
The unitary method is the method used to find the value of a single unit using the value.The number of moles is the given mass by molecular mass.n=m/Mn,m,M is the number of moles, given mass, molecular mass respectivelyTo learn more about moles visit:
brainly.com/question/26416088
#SPJ9
HURRY! NEED WITHIN 10 MINS
Answers
The equation of photosynthesis is shown in the image attached to this question.
What is photosynthesis?The term photosynthesis has to do with a kind of reaction that helps plants to make their own food. The process involves the combination of carbon dioxide and water in the presence of sunlight to give glucose and water only.
We know that the green plants are autotrophic thus they are able to make their own food. The process of photosynthesis produces the food that is passed along the food chain in the ecosystem.
Learn more about photosynthesis:https://brainly.com/question/1388366
#SPJ1