Answers
Motion can simply be defined as the change in position of a body while energy is the ability or capacity to work.
Mechanical energy is one of the forms of energy and is subdivided into potential energy and kinetic energy. These energies are energies that determine whether a body or an object is moving or not.
When a body is in a position, the energy possessed by such body by virtue of its position is known as potential energy while when a body is moving (in motion), the energy possesses by this body by virtue of its motion is the kinetic energy.
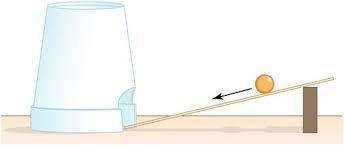
For instance, a ball rolling down an inclined plane is initially at rest at the top of the incline showing that the body has maximum potential energy and zero kinetic energy (since the body is in a position at this point).
As the ball rolls down the incline, it gains momentum and its kinetic energy keeps increasing along the plane while the potential energy keeps decreasing. Once the body gets to the bottom of the plane, the kinetic energy becomes zero while the potential energy is at maximum (because the body is at rest at this point as well).
Related Questions
I have homework to do please I need help with number 7
Answers
The mass number of an atom is defined as the sum of the number of protons and the number of neutrons in that atom.
So, if m is the mass number, p is the number of protons and n is the number of neutrons, we have:
[tex]m=p+n[/tex]We have that:
[tex]\begin{gathered} p=30 \\ n=34 \\ m=30+34=64 \end{gathered}[/tex]So, the mass number is 64.
A balloon is floating around outside your window. The temperature outside is 37 ∘C , and the air pressure is 0.800 atm . Your neighbor, who released the balloon, tells you that he filled it with 3.60 moles of gas. What is the volume of gas inside this balloon?
Answers
Answer:
[tex]114.53\text{ L}[/tex]Explanation:
Here, we want to get the volume of the gas inside the balloon
Mathematically, from the ideal gas equation:
[tex]\begin{gathered} PV\text{ = nRT} \\ V\text{ = }\frac{nRT}{P} \end{gathered}[/tex]where:
P is the pressure which is given as 0.8 atm
V is the volume which we want to calculate
R is the molar gas constant which is 0.0821 L.atm/mol.K
T is the temperature in Celsius which we can convert to K by adding 273 (273 + 37 = 310 K)
n is the number of moles which is 3.6
Substituting the values, we have it that:
[tex]\begin{gathered} \text{ V = }\frac{3.6\text{ }\times0.0821\times310}{0.8} \\ \\ V\text{ = 114.53 L} \end{gathered}[/tex]Ksp= 2×10−19 for LaF3 Calculate the solubility of LaF3 in grams per liter in a solution that is 0.011 M in KF
Answers
Solubility of LaF3: "S"
First, we need to write the reaction of solubility:
LaF3 (s) <=> La+3 + 3 F-
Initial - 0 +0.011 (F-, from KF)
Change +S +3.S
Equilibrium S 0.011 + 3.S
Now, we know:
Ksp = [La³⁺]x[F⁻]³ = 2×10^−19 = S x (0.011+3.S)³
We work with: 2×10^−19 = S x (0.011+3.S)³, and we clear the "S"
=> S = 1.5x10^-13 mol/L x (195.9 g/mol) = 2.93x10^-11 g/L
The molar mass of LaF3 = 195.9 g/mol
Answer: S = 2.93x10^-11 g/L
The reaction of 192g of Fe2O3 with 82.5g of CO produces 72.9 of Fe Fe2O3 + 3CO => 2Fe + 3CO2 Calculate the theoretical yield of iron in grams.
Answers
ANSWER
The theoretical yield of Fe is 109.64 grams
EXPLANATION
Given that;
The mass of Fe2O3 is 192 grams
The mass of CO is 82.5 grams
Follow the steps below to find the theoretical yield of Iron
Steps 1; Find the number of moles of Fe2O3 and CO using the below formula
[tex]\text{ mole = }\frac{\text{ mass}}{\text{ molar mass}}[/tex]Recall, that the molar mass of Fe2O3 and CO are 159.69 g/mol and 28.01 g/mol
[tex]\begin{gathered} \text{ For Fe}_2O_3 \\ \text{ mole = }\frac{\text{ 192}}{\text{ 159.69}} \\ \text{ mole = 1.202 moles} \\ \\ \text{ For CO} \\ \text{ mole = }\frac{\text{ 82.5}}{\text{ 28.01}} \\ \text{ mole = 2.945 moles} \end{gathered}[/tex]Step 2; Determine the limiting raectant of the reaction
To determine the limiting reactant, divide the number of moles by the co-efficient of each reactant
[tex]\begin{gathered} \text{ For Fe}_2O_3 \\ \text{ mol/wt of Fe}_2O_3\text{ = }\frac{\text{ 1.201 }}{\text{ 1}} \\ \text{ mol/wt of Fe}_2O_3\text{ = 1.202 mol} \\ \\ \text{ for CO} \\ \text{ mol/wt of CO = }\frac{\text{ 2.945}}{\text{ 3}} \\ \text{ mol/wt of CO = 0.982 mol} \end{gathered}[/tex]From the calculations, the limiting reactant of the reaction is CO
Step 3; Find the number of moles of Fe using a stoichiometry ratio
Let x represents the number of moles of Fe
[tex]\begin{gathered} \text{ 3 moles CO }\rightarrow\text{ 2 moles Fe} \\ \text{ 2.945 moles CO }\rightarrow\text{ x moles Fe} \\ \text{ cross multiply} \\ \text{ 3 moles CO}\times\text{ x moles Fe = 2 moles Fe }\times\text{ 2.945 moles CO} \\ \text{ x moles Fe = }\frac{\text{ 2 moles Fe}\times2.945mole\cancel{CO}}{3moles\cancel{CO}} \\ \\ \text{ x mole Fe = }\frac{\text{ 2 }\times\text{ 2.945}}{\text{ 3}} \\ \\ \text{ x mole Fe = }\frac{\text{ 5.89}}{\text{ 3}} \\ \text{ x mole Fe = 1.963} \end{gathered}[/tex]The number of moles of Fe is 1.963 moles
Step 4; Find the theoretical yield of Fe
[tex]\begin{gathered} \text{ mole = }\frac{\text{ mass}}{\text{ molar mass}} \\ \text{ cross multiply} \\ \text{ mass = mole }\times\text{ molar mass} \end{gathered}[/tex]Recall, molar mass of Fe is 55.845 g/mol
[tex]\begin{gathered} \text{ mass = 1.963 }\times\text{ 55.845} \\ \text{ mass = 109.64 grams} \end{gathered}[/tex]Therefore, the theoretical yield of Fe is 109.64 grams
Calculate the number of molecules in 3.00 L of CO gas at STP.
Answers
One carbon atom and one oxygen atom bound together by three bonds make up carbon monoxide. The number of molecules in 3.00 L of CO gas at STP is 67.2 liters.
What is carbon monoxide gas ?Colorless, lethal, odorless, tasteless, and combustible, carbon monoxide is a gas that is somewhat less thick than air. One carbon atom and one oxygen atom bound together by three bonds make up carbon monoxide. The oxocarbon family's simplest molecule is this one.
Headache, dizziness, weakness, nausea, upset stomach, vomiting, chest discomfort, and confusion are the most typical signs of CO poisoning. The symptoms of CO are frequently compared to the flu. CO can cause you to lose consciousness or kill you if you breathe it in heavily.
If the gas is at STP,
Then 1 mole = 22.4 liters.
3 moles x 22.4 L/mol
= 67.2 liters.
Thus, The number of molecules in 3.00 L of CO gas at STP is 67.2 liters.
To learn more about carbon monoxide gas, follow the link;
https://brainly.com/question/1238847
#SPJ1
Part A identify club soda as an element, compound, or mixture part B explain why you chose the answer for part A.
Answers
EXPLANATION:
PART
Soda drink is a mixture
PART B
Soda drink is a mixture and not a compound. This is because it contains different constituents and they are mixed together in the right proportion. Also, the composition of the constituents is uniform throughout the mixture.
It was determined that 1.4 x 1024 carbon dioxide molecules are produced when propane is combusted according to the equation below. Calculate the number of moles of propane that was burned.C 3H 8 + 5O 2 ----> 3CO 2 + 4H 2O
Answers
First, we have to convert the number of molecules to the number of moles of CO2 (carbon dioxide), and to do this we can do the Avogadro's number:
The Avogadro's number helps us to determine the number of moles based on the number of molecules or atoms of a compound. This number is 6.022 x 10 ^(23) /mol.
So, the conversion from molecules to moles would be:
[tex]1.4\cdot10^{24}moleculesCO_2\cdot\frac{1molCO_2}{6.022\cdot10^{23}moleculesCO_2}=2.325molCO_2.[/tex]Now, using this data we can calculate the number of moles needed for propane (C3H8).
In the chemical reaction, you can see that 1 mol of propane produces 3 moles of CO2, so the calculation would be:
[tex]2.325molCO_2\cdot\frac{1molC_3H_8}{3molesCO_2}=0.775molC_3H_8.[/tex]The answer is that 0.78 moles of C3H8 were burned and produced 2.325 moles of CO2 which is 1.4 x 10 ^(24) molecules of CO2.
Calculate the percent yield if 1.95 g of Ca(OH)2 reacts with excess HCl to produce 2.50 g of CaCl2. Ca(OH)2 + 2HCl → CaCl2 + 2H2OTheoretical yield?Percent yield?
Answers
The first step is to write a balanced chemical equation.
[tex]Ca(OH)_2+\text{ 2HCl}\rightarrow CaCl_2+2H_2O[/tex]The above equation is already balanced.
Now lets write what we have.
Ca(OH)2 = 1.95g
CaCl2 = 2.50g
We already know that HCl is in excess, meaning Ca(OH)2 is the limiting reagent.
%Yield = (actual yield/theoretical yield) x 100
Lets first calculate the theoretcal yield. To find this we need to first calculate the number of moles of CaCl2.
n = m/M where m is the mass and M is the molar mass of CaCl2
n = 2.50g/110,98 g/mol
n = 0.0225 mol
Theoretical Yield is the amount of product that would have been produced if all of the limiting reagent reacted and it was 100% pure. The limiting reagent is used up first in a reaction and controls the amount of product that can be produced.
The actual yield of CaCl2 we were given, which is 2.50g of CaCl2.
For every 1 mole of Ca(OH)2, 1 mole of CaCl2 is produced
n = 1.95g/74.09 g/mol
n = 0.0263 mol
Therefore the number of moles of CaCl2 = 0.0263 mol
mass of CaCl2 = nM
m = 0.0263 mol x 110,98 g/mol
m = 2.92 g
So the theoretical yield of CaCl2 = 2.92g
percentage yield = (2.50g/2.92)*100
percentage yield = 85.62%
Which molecules separate during the light reaction of photosynthesis?Carbon dioxide and oxygeno Hydrogen and OxygenООHydrogen and Carbon Dioxide
Answers
Answer
Hydrogen and Oxygen
Explanation
During Light reactions of Photosynthesis, the chlorophyll will be activated by light. This light-activated chlorophyll will split the water molecule to release H+ ions and also oxygen. This process is called Photolysis.
Therefore, the correct option to your question is: Hydrogen and Oxygen
What is the percent by mass of bromine in the compound MgBr₂?A) 13.2%B) 43.4%C) 56.6%D) 86.8%
Answers
Answer:
D) 86.8%
Explanation:
It necessary to use the molar mass of Br (80g/mol) and MgBr2 (184g/mol). The MgBr2 is the 100%, so with a mathematical rule of three we can calculate the percent by mass of bromine.
In the molecule of MgBr2 there are 2 bromine, so the total mass of bromine it is 160g (80gx2):
[tex]\begin{gathered} 184g-100\% \\ 160g-x=\frac{160g*100\%}{184g} \\ x=86.9\% \end{gathered}[/tex]Finally, the percent by mass of bromine is 86.9% (86.8%).
What is the 238 U enrichment required for the following applications?
Naturally occurring Uranium: _____ %
Nuclear fuel for power generation: _____ %
Nuclear bombs: _____ %
Answers
Naturally occurring Uranium: 99.2745 %
Nuclear fuel for power generation: 97%
Nuclear bombs: 90 %
With a relative abundance of 99%, uranium-238 (238U or U-238) is the most prevalent isotope of uranium to be discovered in nature. It is non-fissile, unlike uranium-235, hence it cannot support a chain reaction in a thermal-neutron reactor.
A 1000 MWe pressurized water reactor needs about 27 tonnes of uranium per year, or over 18 million fuel pellets contained in more than 50,000 fuel rods. In contrast, a coal power plant of comparable scale would require more than 2.5 million tonnes of coal to generate the same amount of electricity.
15 kilograms: weight of a solid sphere of 100 percent uranium-235, just large enough to reach a critical mass with a beryllium reflector, nuclear weapons normally use a concentration of more than 90 percent.
Hence, the percentages are 99.27, 97 and 90% respectively.
To learn more about uranium 238 refer- https://brainly.com/question/28880128
#SPJ1
Using the equation below, what mass of iron canbe produced from 10.5 grams of aluminum?Al + FeO → Al2O3 + Fe
Answers
32.6 grams
Explanations:Given the reaction between aluminium and ferric oxide as shown;
[tex]2Al+3FeO\rightarrow Al_2O_3+3Fe[/tex]Determine the moles of Aluminium
Mole = Mass/Molar mass
Mole = 10.5/26.98
Mole of Al = 0.3892moles
According to stoichiometry, 2moles of aluminium produced 3 moles of Iron, the moles of Iron needed is given as:
moles of Fe = 3/2 * 0.3892
moles of Fe = 0.5838moles
Determine the mass of iron
Mass of Fe = moles * molar mass
Mass of Fe = 0.5838 * 55.845
Mass of Fe = 32.6grams
Hence mass of iron that can be produced from 10.5 grams of aluminium is 32.6 grams
A 13.5- L scuba diving tank contains a helium-oxygen (heliox) mixture made up of 24.0 g of He and 5.00 g of O2 at 298 K . Calculate the mole fraction of each component in the mixture.
Answers
13.5- L scuba diving tank contains a helium-oxygen (heliox) mixture made up of 24.0 g of He and 5.00 g of O2 at 298 K then mole fraction of each component in the mixture is ˣHe = 1.15 and ˣO₂ = 7
Mole fraction is the ratio of number of moles one component of a solution or other mixture to the total number of moles representing all of the components
Here given data is
Helium = 24.0 g
Oxygen = 5.00 g
Temprature = 298 K
Here we know the mass of both helium and oxygen, in order to obtain the mole fractions we first need the compute the moles by using their atomic masses 4.00 g/mol and 32.00 g/mol respectively as shown below then
ⁿHe = 24.0 g×1 mol He/4.00g He = 6 mol He
ⁿO₂ = 5.00 g×1 mol of O₂/ 32.00 g = 0.15 mol O₂
Then the mole fraction are
ˣHe = ⁿHe/ⁿHe + ⁿO₂ = 6/6 + 0.15
ˣHe = 1.15
ˣO₂ = ⁿO₂/ⁿO₂ + ⁿHe = 0.15/0.15 + 6
ˣO₂ = 7
Know more about mole fraction
https://brainly.com/question/18721537
#SPJ1
Local winds are produced by geographic freatures true or false
Answers
Explanation:
Hey! The answer is True!
\Which of the following should be broken into ions when writing a complete ionic equation? Select all that apply.
Answers
Step 1 - How to tell whether a given substance is ionic or covalent
To decide whether a substance is ionic or covalent, we must look for metals in its formula. If the substance is composed of both metals and non metals, it is an ionic susbtance.
If it's, on the other hand, composed solely by non-metals, it is a covalent substance.
Covalent substances do not produce ions in aqueous solution. Therefore, they can't be "broken into ions".
Step 2 - Analysing the given substances
H2O --> composed of H and O; both non-metals, so covalent
LiF --> composed of a metal (Li) and a non-metal (F), so ionic
K3PO4 --> composed of a metal (K) and non-metals (P, O), so ionic
AgCl --> composed of a metal (Ag) and non-metal (Cl), so ionic
Step 3 - Answering the exercise
All substances except H2O should be broken into ions when writing a complete ionic equation.
Answer: all except H2O
write a flow diagram or dimensional analysis scheme from % Mass to Molarity
Answers
To convert %Mass to Molarity, we have to know two things: the molecular mass of the solute and the density of the solution.
The first step is to convert the mass of the solute to moles using the molecular mass.
The second step is to conver the mass of the solution to liters of solution using the density.
Finally, the last step is to simplify the obtained result.
PLEASE HELPPPP CHEMISTRY
Answers
There are 1.02391×1024 no. of atoms in 1.70 moles of Calcium (Ca)
What is a Avogadro number?The approximate number of nucleons (protons or neutrons) in a gram of common stuff is known as the Avogadro number. 1 mole of any material contains the no of particles equal to the Avogadro number, that is, 6.023×1023particles.
What is Calcium?The chemical element calcium has the atomic number 20 and the letter Ca as its symbol. Calcium is an alkaline earth metal that reacts with air to create a black oxide-nitride coating. Its heavier homologues barium and strontium are most similar to it in terms of physical and chemical characteristics.
Now,
Acc. to the Avogadro Number principal every 1 mole of any material contains the no of particles equal to the Avogadro number, that is, 6.023×1023particles.
So, the given amount of Calcium contains atom as follows:
1.70×6.023×1023 =1.02391×1024 atoms
Hence, There are 1.02391×1024 no. of atoms in 1.70 moles of Calcium (Ca)
To know more about Avogadro Number visit
https://brainly.com/question/28834341
#SPJ1
Determine if this is a property of acids, bases, or neutral solutions: Feels slippery to the touch.
Answers
Although there are neutral solutions that feel slipery, this is not a characteristic of all neutral solutions.
Acid solutions normally feels similar to neutral solutions, just wet.
However, base solutions has the characteristics of feeling slippery.
So, the most correct answer would be base
Answer: Bases
Explanation:
Bases have a slippery feel. Many soaps and detergents contain bases. Strong bases are able to react with the fatty acids and oils that naturally occur on the surface of your skin. The product of the reaction (which is known as saponification) is effectively a soap, which is why it feels slippery.
Can I find a tutor to help me with question?
Answers
Answer:
Hafnium is more likely to be the identity of the substance.
Explanation:
The given information about the substance is:
- Mass: 46.9g
- Volume: 3.5cm^3
- Solid at room temperature (23°C)
We can calculate the density of the substance by replacing the values of mass and volume in the density formula:
[tex]\begin{gathered} Density=\frac{mass}{volume} \\ . \\ Density=\frac{46.9g}{3.5cm^3} \\ . \\ Density=13.4\frac{g}{cm^3} \end{gathered}[/tex]Now we know that the density of the substance is 13.4g/cm^3.
At this point, the substance could be mercury or hafnium, because they have a similar density, but we also know that the substance is solid at 23°C, so since hafnium has a melting point of 2,233°C, it is more likely to be the identity of the substance.,
A gas occupies 3.8 L at -18° C and 975. torr. What volume would this gas occupy at STP?
Answers
To solve the question we will assume that the gas behaves like an ideal gas, that is to say, that there is no interaction between the molecules. Assuming ideal gas we can apply the following equation:
[tex]PV=nRT[/tex]Where,
P is the pressure of the gas
V is the volume of the gas
n is the number of moles
R is a constant
T is the temperature
Now, we have two states, an initial state, and a final state. The conditions for each state will be.
Initial state (1)
P1=975Torr=1.28atm
V1=3.8L
T1=-18°C=255.15K
Final state(2), STP conditions
P2=1atm
T2=273.15K
V2=?
We will assume that the number of moles remains constant, so the nR term of the first equation will be constant. For each state, we will have:
[tex]\begin{gathered} \frac{P_1V_1}{T_1}=nR \\ \frac{P_2V_2}{T_2}=nR \end{gathered}[/tex]Since nR is the same for both states, we can equate the equations and solve for V2:
[tex]\begin{gathered} \frac{P_{2}V_{2}}{T_{2}}=\frac{P_1V_1}{T_1} \\ V_2=\frac{P_{1}V_{1}}{T_{1}}\times\frac{T_2}{P_2} \end{gathered}[/tex]We replace the known values:
[tex]V_2=\frac{1.28atm\times3.8L}{255.15K}\times\frac{273.15K}{1atm}=5.2L[/tex]At STP conditions the gas would occupy 5.2L. First option
A 16%(m/m) solution contains 10 grams of NaOH. It also contains ___ grams of water.
Answers
1) List the known and unknown quantities.
Concentration: 16%(m/m).
Mass of solute: 10 g NaOH.
Mass of solvent: unknown.
2) Set the equation.
Mass percent.
[tex]Mass\text{ }\%=\frac{grams\text{ }of\text{ }solute}{grams\text{ }of\text{ }solvent}*100[/tex]3) Plug in the known values and solve for grams of solvent (water).
[tex]16\%=\frac{10\text{ }g\text{ }NaOH}{grams\text{ }of\text{ }solvent}*100[/tex].
[tex]grams\text{ }of\text{ }solvent=\frac{10\text{ }g\text{ }NaOH}{16}\times100[/tex][tex]grams\text{ }of\text{ }solvent=62.5\text{ }g\text{ }H_2O[/tex]The 16%(m/m) solution contains 62.5 g H2O.
.
If an ideal gas does not really exist, why do scientists use this this concept?
Answers
The ideal gas model was created to summarize some laws and study the behaviour of the gases, that depends on the properties.
It is really helpful and it is the first approach to the real behaviour of the gases, because all the other equations that came out after it, they have a basis on it. Historically, the ideal gas model was one of the first and most suitable ways to describe and calculate the properties of the gases.
It is also important that it's one of the easiest ways to relate the properties that are in the model, and it's very useful under certain conditions (specially temperature and pressure).
10. Which of the following would speed up the rate of a the reaction A(s) + B(aq) → AB(aq) +heata.Add more of reactant Ab. Add more of product ABC. Crush reactant A into a powderd.Cool the reaction vessel
Answers
In this question, we need to determine which option can speed up the rate of a chemical reaction, and there are a few ways possible in order to speed up a reaction, usually we have: Increasing temperature, increasing surface area, increasing the concentration of reactants
In this case, increasing temperature would not make the reaction occur faster, since the reaction is exothermic, therefore there is no need to input energy for the reaction to occur
Increasing the concentration of reactants, is the option A, but there is a small problem, A is a solid compound and B is an aqueous compound, if we add more A, we can speed up the reaction but we can also turn the extra amount of A insoluble, which would not make the reaction to occur faster
The best way, in this case, is to crush reactant A into powder, because this would make the surface area of A to increase, making A and B interact more and then the reaction would occur faster
The best option will be letter C
Hi I need help with this question please and thank you
Answers
Let's see an illustration of a water molecule:
You can see that the oxygen has two pairs of electrons free representing a partial negative charge. These partial negative charges don't allow the molecule has a linear structure. All of the electron pairs—shared and unshared—repel each other and that's why the water molecule has a bent shape, because of these unshared electrons of oxygen.
24.How is a supersaturated solution prepared?Select one:a. Heat a solution to the boiling point and continue to boil for 15 minutes.b. Add solute to a saturated solution and heat until all of the solute dissolves. Then, slowly cool to the original temperature.c. Add solute to cold solvent and then heat the solution to room temperature.d. Stir the solution until all of the solid dissolves and then heat the solution.
Answers
Answer
b. Add solute to a saturated solution and heat until all of the solute dissolves. Then, slowly cool to the original temperature.
Explanation
A supersaturated solution contains more dissolved solute than required for preparing a saturated solution and can be prepared by heating a saturated solution, adding more solute, and then cooling it gently. Excess dissolved solute crystallizes by seeding supersaturated solution with a few crystals of the solute.
Therefore, a supersaturated solution is prepared by:
b. Add solute to a saturated solution and heat until all of the solute dissolves. Then, slowly cool to the original temperature.
Answer the question with the choices Which is not a biotic factor that influences the relationships of organisms in ecosystem?A-Competition among predatorsB- A disease caused by bacteriaC-Invasive speciesD-Weather
Answers
Step 1 - Understanding what is a biotic factor
Biotic, as well as other words with "bio" in them, is related to living organisms. A biotic factot is, therefore, a factor which depends on living organisms. When living organisms shape their environment, as well as other living beings environments, we call it a "biotic factor".
Step 2 - Using the definition to solve the exercise
a) Competition among predators: since this is a consequence of the struggling between two or more living beings, the outcome of it is surely a biotic factor. Individuals can die because they could not find enough food due to competition. A population could thus be modified by this biotic factor, for example.
b) A disease caused by bacteria: a bacteria is a living being and a disease can disastrously kill an entire population, radically changing the ecological relations and the environment. This is another biotic factor.
c) Invasive species: this is also caused by living beings. When a specie invades another species territory, there may be fight among them or just resources sharing. Either way, the ecological relations will be drastically modified. This accounts for a biotic effect as well.
d) weather: this is not solely caused by living beings. In fact, it depends rather poorly on living beings, and much more on Earth's temperature, geographical location an so on. Since the changes caused by weather are not from a living source, this is not a biotic factor.
The answer is thus item d.
Lidocaine, a widely used local anesthetic, it’s available as a 2.0% (w/v) solution for injection. Calculate the volume of the solution that contains 60 mg of lidocaine. Be sure your answer has a unit symbol and is rounded to the correct number of significant digits.
Answers
Givens.
• Lidocaine is available as a 2.0% (w/v) solution.
,• The mass of solute is 60. mg of lidocaine.
To find the volume of this solution, we use the following equation.
[tex](\frac{w}{v})=\frac{Mass\text{ of solute}}{Volume\text{ of solution}}[/tex]Use the given magnitudes and solve for volume.
[tex]\begin{gathered} 0.02=\frac{60mg}{V} \\ V=\frac{60mg}{0.02} \end{gathered}[/tex]But, we need to use grams.
[tex]V=\frac{0.060g}{0.02}=3[/tex]Therefore, the volume of this solution is 3.0 mL.
2 H₂O + electricity → 2 H₂ + O₂15. In which type of cell would this reaction most likely occur?(1) a chemical cell, because it is exothermic(2) an electrolytic cell, because it is exothermic(3) a chemical cell, because it is endothermic(4) an electrolytic cell, because it is endothermic
Answers
Answer:
(4) an electrolytic cell, because it is endothermic.
Explanation:
You can see that we're using electricity to carry out the reaction, so this reaction is occuring in an electrolytic cell.
Remember that electricity is a form of energy. In an exothermic process, the energy is released but in an endothermic process, the energy is being absorb, and the process would required energy.
As you can note, this reaction requires electricity, it required energy, so this would be an endothermic process.
The answer would be (4) an electrolytic cell, because it is endothermic.
edAnswersweredQuestion 6How many H atoms are in 3.4 mol C21H23NO5?1.3 x 10³2.9 x 1042.0 x 10244.7 x 10254.7 x1025. This was the correct answer.↓0/1 pts0/1 ptc
Answers
Given the following parameters
moles of C21H23NO5 = 3.4 moles
Since there are 23 atoms of hydrogen in the compound, hence the moles of hydrogen in the compound is given as:
[tex]\begin{gathered} moles\text{ of H}=23\times3.4 \\ moles\text{ of H}=78.2moles \end{gathered}[/tex]According to the Avogadro's constant
[tex]1mole\text{ of a substance}=6.02\times10^{23}atoms[/tex]Convert the moles of hydrogen to atoms
[tex]\begin{gathered} atoms\text{ of H}=78.2\times6.02\times10^{23} \\ atoms\text{ of H}=470.764\times10^{23} \\ atoms\text{ of H}=4.70764\times10^{25} \\ atoms\text{ of H}=4.7\times10^{25}atoms \end{gathered}[/tex]6.The ability to do which of the following is a chemical property of a substance?Select one:a. React with oxygen.b. Conduct electricity.c. Be drawn into a wire.d. Be worked into different shapes.
Answers
Answer:
[tex]A\text{ : React with oxygen}[/tex]Explanation:
Here, we want to select the option that represents a chemical property
A chemical property is one in which there is a chemical change
Asides from the first option, the other are physical
By reacting with oxygen, entirely new substances are formed