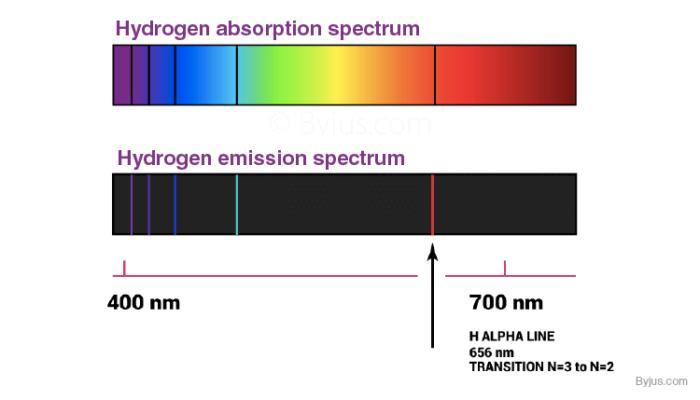
Explain why the spectrum produced by a 1-gram sample of element z would have the same spectral lines at the same wavelengths as the spectrum produced by a 2-gram sample of element z.
Answers
The spectrum produced by a 1-gram sample of element z would have the same spectral lines at the same wavelengths as the spectrum produced by a 2-gram sample of element z because the position of the spectral lines does not depend on the amount of mass. but of the type of atom or molecule that produces them.
What are spectral lines?They are dark or bright lines in a uniform and continuous spectrum, the result of an excess or deficiency of photons in a narrow range of frequencies, compared to other nearby frequencies.
Spectral lines correspond to a certain atomDue to the intrinsic property that each atom has to emit a single spectral line, these lines have been very useful to identify the chemical composition of any medium that allows light to pass through it.
The use of spectroscopyThe spectral lines also depend on the physical conditions of the gas, hence they are used to determine the physical characteristics and chemical composition of stars and other celestial bodies, for which there is no other method of analysis.
Learn more about spectral lines at https://brainly.com/question/27494244
#SPJ4
Related Questions
phosphoric acid is tribasic, with pka's of 2.14, 6.86, and 12.4. the ionic form that predominates at ph 7.2 is:
Answers
Phosphoric acid is tribasic, with pka's of 2.14, 6.86, and 12.4. the ion form that predominates at ph 7.2 is Phosphoric acid (H3PO4) is tribasic, with pKa's of 2.14, 6.86, and 12.4.
What is ion?
An ion is an atom or group of atoms in which the number of electrons, s, differs from the number of protons, s. If the number of electrons is less than the number of protons, the particle is a positive ion, also called a positive ion.
Therefore, Phosphoric acid is tribasic, with pka's of 2.14, 6.86, and 12.4. the ion form that predominates at ph 7.2 is Phosphoric acid (H3PO4) is tribasic, with pKa's of 2.14, 6.86, and 12.4.
To learn more about ion refer the given link:-
https://brainly.com/question/15162251
#SPJ4
place the components of the electron‑transport chain to outline the flow of electrons from nadh to o2.
Answers
The components of the electron‑transport chain to outline the flow of electrons from NADH to O₂ is :
NADH --> NADH dehydrogenase ---> ubiquinone ---> cytochrome b-c1 complex ----> cytochrome c ---> cytochrome oxidase complex ---> oxygen ( O₂ ).
There are three respiratory enzymes complex in the respiratory chain in the inner mitochondrial membrane. The electron transport chain passes on the way from NADH to O₂ n order as given below:
NADH --> NADH dehydrogenase ---> ubiquinone ---> cytochrome b-c1 complex ----> cytochrome c ---> cytochrome oxidase complex ---> oxygen ( O₂ ). in the electron transport chain the oxygen is the final electron acceptor. the NADH is donor of the electrons.
To learn more about electron transport here
https://brainly.com/question/29432698
#SPJ4
What would happen if you used equal masses of all the compounds? For example, 2.00 grams as opposed to equal moles of each compound? O Equal masses would produce different amounts of CO, Equal masses would produce the same amount of CO2 Equal masses would show extreme variation and be difficult to measure in the lab.
Answers
Equal masses have different amounts of CO, but equal masses have the same amount of CO2. Equivalent masses are highly variable and difficult to measure in the laboratory.
Density is a measure that combines matter, mass, and volume. Density is determined by dividing the mass of a substance by its volume. Grams are commonly used to measure mass. As for volume, solids are measured in cubic centimeters or cubic meters, liquids in liters or milliliters.
The two items which have same loads head closer to every different at same speeds after which stick together. The items come to relaxation after sticking together, preserving momentum however now no longer kinetic electricity when they collide. Some of the electricity of movement receives transformed to thermal electricity, or heat.
Read more about mass:
https://brainly.com/question/13130538
#SPJ4
Why must lithium levels be carefully monitored in individuals who take this medication?
Answers
Lithium levels be carefully monitored in individuals who take this medication if levels are too high, you may experience lithium toxicity
The lightest of the solid elements is lithium (Li), an element belonging to Group 1 (Ia) of the periodic table. A number of its alloys and compounds, as well as the soft, white, and lustrous metal itself, are produced on an industrial scale. By electrolyzing a fused mixture of lithium and potassium chlorides, lithium metal is created. Lower-temperature operation of the electrolysis is made possible by the mixture's lower melting point (400-420 °C, or 750-790 °F) compared to that of pure lithium chloride (610 °C, or 1,130 °F). Lithium is deposited at a purity level higher than 97 percent because the voltage at which decomposition of lithium chloride occurs is lower than that of potassium chloride. Lithium is produced electrolytically using graphite anodes.
To know more about Lithium visit : https://brainly.com/question/1439744
#SPJ4
what conditions must be true for the michaelis-menten formality to be applicable to an enzymatic reaction?
Answers
The conditions are:
1. An enzyme must catalyze the reaction.
2. The reaction must involve a substrate and a product.
3. The substrate concentration must determine the reaction rate.
4. The reaction must follow first-order kinetics.
5. The reaction must be reversible.
6. The enzyme must be in a steady state with respect to the substrate.
7. The enzyme must not be saturated with substrate.
8. The rate of the reaction must be proportional to the amount of enzyme present.
The Michaelis-Menten formality is a mathematical model used to describe the kinetics of an enzymatic reaction, and it is applicable when the following conditions are met:
1. The reaction must be a reversible, single-substrate reaction, meaning that a single substrate binds to the enzyme, forms an enzyme-substrate complex, and then the product is released.
2. The reaction must be at steady state, meaning the rate of formation of the enzyme-substrate complex is equal to the rate of its breakdown.
3. The reaction must be at equilibrium, meaning the concentrations of the enzyme, substrate, and product remain constant.
4. The reaction must follow a simple kinetic mechanism, meaning the rate of the reaction is determined solely by the substrate concentration.
5. The reaction must have an initial rate that is linearly proportional to the enzyme concentration.
6. The reaction must have a Michaelis-Menten constant (Km) that is independent of the enzyme concentration.
7. The reaction must have an enzyme that is not inhibited by the product.
8. There must be a single, rate-limiting step.
To know more about concentration:
brainly.com/question/10725862
#SPJ4
why did we choose these particular salts to test how the soap reacts with hard water?
Answers
Epsom Salt is used to test how the soap reacts with hard water because it forms precipitates with hard water
Epsom salt also known as magnesium sulfate is used to check the reaction of soap with hard water because it separates into ions giving us Mg2+ and SO4 2- ions.
Now, when we add soap to this water, soap combines with the magnesium ions and form solid particles that accumulate in clusters that become insoluble. These clusters are called precipitates which can be observed physically.
Hence, it proves the hardness of water and explains why particular salts to test how the soap reacts with hard water.
You can learn more about how soap reacts with hard water from
https://brainly.com/question/29823234
#SPJ4
Does STP mean one mole?
Answers
Answer:
No. STP is an acronym for Standard Temperature Pressure. There is a useful conversion factor that involves one mole: One mole of any gas osscupies 22.4 liters.
Explanation:
22.4 liters/mole of any gas at STP (Standard Temperatue Pressure)
a sample initially contains 5.2 moles of a radioactive isotope. how much of the sample remains after four half-lives?
Answers
A sample initially contains 5.2 moles of a radioactive isotope. 0.325 moles much of the sample remains after four half-lives.
What is radioactive isotope?A chemical element in an unstable state that emits radiation as it decomposes and becomes more stable. Radioisotopes can be created in a lab or in the natural world. They are utilised in imaging studies and therapy in medicine. Also known as a radionuclide.
There are several uses for radioactive isotopes. Cobalt-60, for instance, is frequently used as a radiation source in medicine to halt the spread of cancer. For diagnostic reasons and in studies on metabolic processes, other radioactive isotopes are utilized as tracers.
After each half life, substance reduces by half after n half life:
A/A₀ = (1/2)ⁿ
Given that, A₀ = 5.2 moles
no. of half-lives (n) = 4
Thus,
A/5.2 = (1/2)⁴
or, A/5.2 = 0.0625
or, A = 0.0625 × 5.2
or, A = 0.325 moles.
To know more about radioactive isotope refer to:
https://brainly.com/question/28039996
#SPJ1
pressure has little effect on the solubility of liquids and solids because they are almost incompressible. t or f
Answers
False. Pressure has a direct impact on the solubility of liquids and solids. Increasing pressure raises the solubility of a given substance, while decreasing pressure reduces it. This is because higher pressure forces molecules closer together, making it easier for them to dissolve in a solvent.
The Impact of Pressure on the Solubility of Liquids and SolidsThe effect of pressure on the solubility of liquids and solids is an important factor to consider in many industrial processes. When the pressure is increased, the solubility of a given substance increases as well, due to the increased force between molecules which makes it easier for them to dissolve in a solvent.
Learn more Solute and solvent: https://brainly.com/question/25326161
#SPJ4
select sulfur hexafluoride, sf6, and set the mass to 4.00 g. what amount (in moles) of sf6 does 4.00 g of sf6 represent?
Answers
6.022x10^23 molecules are contained by any compound consisting of 1 mole. Similarly 1 molecule of sulfur hexafluoride, SF6 contains 0.00425 moles.
An inorganic chemical having the formula SF6 is sulfur hexafluoride. It is a gas that has no color, no smell, is not combustible, and is not harmful. Six fluorine atoms are joined to a sulfur atom in the middle of the compound SF 6, which has an octahedral structure. It is a molecule with a high valent. An artificial, odorless gas called SF6 is utilized in the energy sector to maintain the security and dependability of networks. Because it is highly stable, non-toxic, inflammable, and electronegative, it won't combine with other substances that would change how it behaves or how effective it is.
Number of moles =compound mass/molar mass
Given mass of SF6 is 4g
Molar mass of SF6 sulfur hexafluoride is 1xmolar mass of sulfur + 6xmolar mass of fluorine = 1x32 +6x19 = 146g
number of moles in SF6 = 4/146 = 0.027
To learn more about sulfur hexafluoride click here https://brainly.com/question/24186669
#SPJ4
what is the theoretical yield for this reaction in grams if you start with 0.240 ml of 1-methylcyclohexene and use the reagents in the amounts described in the procedure? report your answer to three decimal places but do not include the unit.
Answers
0.391 is the theoretical yield for this reaction in grams if we start with 0.240 ml of 1-methylcyclohexene and use the reagents.
Theoretical yield is the amount of a product that results from the complete conversion of the limiting reactant in a chemical reaction.
The maximum mass of a product that can be produced in a chemical process is known as the theoretical yield. The relative formula mass of the product, the mass and relative formula mass of the limiting reactant, and the balanced chemical equation can all be used to compute it.
A clear, colorless liquid with a smell resembling petroleum is what methylcyclohexane looks like. 25 °F flash point Water-insoluble and less dense than water.
To know more about mass, visit:
https://brainly.com/question/28021242
#SPJ4
a solution is prepared by bubbling nh3 in water until 25 g of nh3 dissolves. the final solution weighs 250 g and the density of the solution is 0.974 g/ml. what is the mole fraction of nh3 in the solution?
Answers
The mole fraction of the NH₃ in the solution is 0.0955.
given that :
mass of the NH₃ = 25 g
mass of the H₂O = 250 g
moles of the NH₃ = 25 g/ 17.03 g /mol
= 1.468 mol
moles of the H₂O = 250 g/ 18 g /mol
= 13.88 mol
total moles = 15.36 mol
the mole fraction of NH₃ = moles of NH₃ / total moles
= 1.468 / 15.36
= 0.0955
Thus, A solution is prepared by bubbling NH₃ in water until 25 g of nh3 dissolves. the final solution weighs 250 g and the density of the solution is 0.974 g/ml. the mole fraction of NH₃ in the solution is 0.0955.
To learn more about mole fraction here
https://brainly.com/question/15883465
#SPJ4
The following ions contain the same number of electrons. Rank them in order of decreasing ionic radii!
Sc3+, P3-, Cl-, Ca2+, K+, S2-
Answers
The ionic radii for the given ions are respectively described:
[tex]Sc^{3+}[/tex] = 74.5 pm
[tex]P^{3-}[/tex] = 212 pm
[tex]Cl^{-}[/tex] = 181 pm
[tex]Ca^{2+}[/tex] = 100 pm
[tex]K^{+}[/tex] = 138 pm
[tex]S^{2-}[/tex] = 184 pm
Therefore, to put them in decreasing order according to the ionic radii, then the order would be:
[tex]P^{3-}, S^{2-}, Cl^{-}, K^{+}, Ca^{2+}, Sc^{3+}[/tex]
The radius of a single ion in an ionic crystal is denoted by the symbol "[tex]r_{ion}[/tex]." The distance between ions in a crystal lattice is equal to the sum of the radii of the cation and anion, despite the fact that neither atoms nor ions have clear boundaries. The radius of an ion is commonly expressed in picometers (pm) or angstroms (Å), where 1 Equals 100 pm.
Coordination number, spin state, and other characteristics affect ionic radius. Ionic radius readings are transferrable enough to identify periodic trends. Ionic radii rise with group descent, like other atomic radii. High-spin ions are larger than low-spin ones, and coordination number enhances ionic size. Positive charge decreases ionic radius, while negative charge increases it.
To know more about ionic radius:
https://brainly.com/question/8137711
#SPJ4
Arrange the organic compounds from most soluble in water to least soluble in water.
a. CH4 b. CH3OH c. CH3OCH
Answers
The substance that is most soluble in water among the other substances is ethylene glycol (HOCH2CH2OH). The two hydroxy groups in ethylene glycol combine with water to produce hydrogen bonds.
Which organic molecule dissolves the least easily in water?
Octane is the chemical compound that dissolves the least readily in water. Because only two hydrocarbons, carbon and hydrogen, make up octane, it is a nonpolar molecule due to the comparable electronegativities (ENs) of the atoms. The least soluble fuel in water will be octane because water is a polar solvent.
Is CH3OCH3 water soluble?
Dimethyl ether (CH3OCH3) (C H 3 O C H 3) and water molecules also form hydrogen bonds. As a result, it is also soluble in water, but due to the bulky methyl group in dimethyl ether, there is less hydrogen bonding.
To know more about soluble in water visit;
https://brainly.com/question/5173732
#SPJ4
A flask has a mass of 78. 23 g when empty and 593. 63 g when filled with water. When the same flask is filled with concentrated sulfuric acid, h2so4, its mass is 1026. 57 g. What is the density of concentrated sulfuric acid in g/cm3? (assume water has a density of 1. 00 g/cm3 at the temperature of the measurement. ).
Answers
The density of concentrated sulfuric acid that filled into a flask with 1026.57g mass is 1.84 g/cm^3.
It is the substance's mass per unit of volume. The symbol that used for density is ρ and it expressed in units of grams per cubic centimetre.
How to calculate the density of concentrated sulfuric acid, H2SO4?
Volume of Flask = 593.63 - 78.23 = 515.4g of water
1g of water = 1cm^3 so the volume is 515.4cm^3
Mass = 1026.57 - 78.23 = 948.34g
Density of sulfuric acid = Mass / Volume
= 948.34 / 515.4
= 1.84 g / cm^3
Therefore, the density of concentrated sulfuric acid is 1.84 g/cm^3
Learn more about density https://brainly.com/question/952755
#SPJ4
the volume of 350. ml of gas at 25°c is decreased to 135 ml at constant pressure. what is the final temperature of the gas?
Answers
The decrease in the volume of gas at constant pressure results in the final temperature of the gas is 115.05 K.
The Charles law states that with the gas constant pressure there has been a proportional relationship between the volume and temperature.
Gas is a state of matter that has no fixed shape and no fixed volume. Gases have a lower density than other states of matter, such as solids and liquids. There is a great deal of empty space between particles, which have a lot of kinetic energy and aren't particularly attracted to one another.
Kinetic electricity is the energy an object has due to its movement. If we need to boost up an item, then we ought to follow a pressure. applying a force calls for us to do paintings. After work has been achieved, energy has been transferred to the item, and the object might be shifting with a brand new consistent pace.
Learn more about gas here :-
brainly.com/question/24719118
#SPJ4
what is the molar mass of potassium osmium manganese
Answers
The molar mass of the Potassium, Osmium, and Manganese are 39.10 g/mol, 190.23 g/mol, and 54.94 g/mol respectively.
What is the molar mass?The molar mass can be described as the mass of one mole of a substance. In one mole, there are 6.023 × 10²³ molecules or atoms present and this number is known as Avogadro’s number.
The molar mass of a chemical compound is determined by the sum of the atomic mass of each substance in the molecule.
The molar mass can be described as an intensive property of the substance as it does not depend on the size of the sample. In the S.I. system, the unit of molar mass can be represented as kg/mol. Molar masses are almost always represented in g/mol.
Learn more about molar mass, here:
brainly.com/question/12127540
#SPJ1
what is a sign in the chemical reaction that the acid/base is stronger than the conjugate acid/conjugate base in a chemical reaction?
Answers
If the acid and base are the reactants, then the conjugate acid and conjugate base are the products
What is Conjugate Acid?
There is a conjugate base for every acid. Every base has an acid conjugate. When acids react, they "give" H+. When they separate in water, this is most clearly visible:
This illustration uses sulfuric acid (H
Due to the fact that it "donates" H + to the water, 2 S O 4) is an acid. It turns into the hydrogen sulfite ion, or H S O 4—the conjugate base of sulfuric acid.
A conjugate acid differs from the base it was generated from in that it has one additional H atom and one more + charge
The conjugate base is what is left over after the acid has finished doing its work and lost a proton.
The conjugate acid is what is produced after the initial base has finished its work. take in a proton.
It truly is that easy.
KOH + HCl results in K+, Cl-, and H2O.
Cl(-) is the conjugate base of HCl in the example above, while water is the conjugate acid of hydroxide.
Learn more about Conjugate Acid from given link
https://brainly.com/question/12584785
#SPJ4
a chemist, running tests on an unknown sample from an illegal waste dump, isolates 18 grams of what he suspects is a radioactive element. in order to help identify the element, he would like to know the half-life. he determines that after 50 days only 5 grams of the original element remains. what is the half-life of this mystery element? round your answer to two decimal places, if necessary.
Answers
The half-life. he determines that after 50 days only 5 grams of the original element remains The half-life of this mystery element 26.99 days.
The expression for the half life is given as :
5 = 18(1/2)^50/t
log( 5/18 ) = 50 / t log( 1/2)
-0.5575 = 50 / t ( - 0.3010)
t = 15.05 / 0.5575
t = 26.99 days
Thus, A chemist, running tests on an unknown sample from an illegal waste dump, isolates 18 grams of what he suspects is a radioactive element. in order to help identify the element, he would like to know the half-life. he determines that after 50 days only 5 grams of the original element remains. the half-life of this mystery element is 26.99 days.
To learn more about half life here
https://brainly.com/question/6770013
#SPJ4
How many grams of chlorine gas are found in a 12.7 L sample at STP.
Answers
Considering the definition of STP conditions, a mass of 20.10 grams of chlorine gas is found in a 12.7 L sample at STP.
STP conditionsSTP conditions refer to standard temperature and pressure, using 1 atmosphere and 0 °C as reference values for gases. Under these conditions, 1 mole of any gas occupies an approximate volume of 22.4 liters.
Molar massThe molar mass of substance is the amount of mass that a substance contains in one mole.
Mass of chlorine gasIn this case, you can apply the following rule of three: if by definition of STP conditions 22.4 liters of chlorine is occupied by 1 mole of chlorine, 12.7 liters is occupied by how many moles of chlorine?
moles= (12.7 liters× 1 mole)÷ 22.4 liters
moles= 0.567 moles
The molar mass of chlorine is 35.45 g/mole.
Now, you can apply the following rule of three: If by definition of molar mass 1 mole of chlorine has a mass of 35.45 grams, 0.567 moles of chlorine has how much mass?
mass= (0.567 moles× 35.45 grams)÷ 1 mole
mass= 20.10 grams
Finally, a mass of 20.10 grams of chlorine gas is present.
Learn more about STP and molar mass:
brainly.com/question/3773297
brainly.com/question/9901446
brainly.com/question/12695086
#SPJ1
The national fire protection association (nfipa) 704 diamond label system helps firefighters and employees easily recognize the hazardous substances stored on the site.
Answers
The (NFIPA) 704 diamond label system helps firefighters and employees easily recognize the hazardous substances stored on the site is correct statement.
The categories of hazardous materials stored on the site are easily recognised by firefighters and staff thanks to National Fire Protection Association (NFIPA) 704 standards. The National Fire Protection Association has published it to supplement the labelling system for emergency responders and to help those who work in regular environments. Materials Safety Data Sheet refers to the document created by the manufacturer that lists the risks associated with a product. Hazardous materials are defined as substances or materials that, when transported, stored, or used in commerce, could pose an unreasonably high risk to one's health, safety, or property.
To know more about National Fire Protection Association visit : https://brainly.com/question/11251534
#SPJ4
Which of the following is a sign that a chemical change has occurred?
Group of answer choices
glass changes shape when it falls and breaks
water produces a gas when it is placed on a hot stovetop
a firework explodes releasing heat, sound, and light energy
a substance becomes cold when it is placed in the freezer
Answers
given the electronegativity values of c (2.5), n (3.0), o (3.5), and s (2.5), which of the following molecules have polar bonds? question 5 options: a) carbon dioxide, co2 b) none of the above c) sulfur dioxide, so2 d) all of the above e) nitrogen dioxide, no2
Answers
Polar covalent bonds with an electronegativity difference of at least 0.5 are present in every one of the above compounds.
The sharing of electrons between two atoms defines covalent bonds. The polarity of a certain covalent bond is determined by the difference in electronegativity between the atoms. Between atoms with identical electronegativities, a non-polar covalent link is created, whereas between atoms with electronegativities ranging from 0.5 to 1.7, a polar covalent bond is created. Carbon and oxygen are bound together. Bund is hence polar. since their electro negativities differ. Different electronegativities apply to O and S. Therefore, the bond is polar and N and O have different electronegativities, Polar connection is also. Over a time, electronegativity increases from left to right. When the valence shell is nearly full, an atom's pull on the bonding pair's electrons is greatest.
To learn more about electronegativity click here https://brainly.com/question/17762711
#SPJ4
Choose the combination of factors that would lead to the greatest oxygen unloading from hemoglobin.
a. Low pH, high temperature, high Pcoz, high 2,3-BPG b. Low pH, high temperature, low Pcos, high 2,3-BPG High pH, c. low temperature, low Pcoz, low 2,3-BPG d. High pH, high temperature, high Pcoz, low 2,3-BPG
Answers
The combination factors that would lead to greatest oxygen unloading from hemoglobin is Low pH, high temperature, high PCO2, high 2,3-BPG.
Group 16 of the periodic table, also known as the oxygen group, contains the non-metallic chemical element oxygen (O). All living things require oxygen, a colorless, flavorless, and odorless gas. Plants utilize the carbon dioxide that animals have absorbed and converted into by using it as a source of carbon and releasing the oxygen back into the environment. In addition to reactions that remove elements from their combinations with one another, oxygen can react with almost every other element to form compounds. These reactions are referred to as combustions, and they frequently result in the production of heat and light. Water is the most crucial component.
To know more about oxygen visit : brainly.com/question/11587330
#SPJ4
which of the molecules is the major regulator of oxygen consumption during oxidative phosphorylation?
Answers
The primary method by which aerobic organisms produce ATP is by oxidative phosphorylation. As the main electron carriers that transfer electrons to oxygen, the final acceptor, during the oxidation of fuel molecules, FADH2 and NADH play a crucial role (O2).
What is oxidative phosphorylation?
The generation of ATP from ADP and Pi is made easier in the mitochondrion thanks to the proton gradient. Because the conversion of ADP to ATP relies on the oxidative reactions taking place in the mitochondria, this process is also known as oxidative phosphorylation.
To learn more about oxidative phosphorylation
Here: https://brainly.com/question/13254827
#SPJ4
draw the alkyne formed when 2,3‑dichloropentane is treated with an excess of strong base such as sodium amide.
Answers
The same catalysts used in alkene hydrogenation—platinum, palladium, nickel, and rhodium—are utilized in the catalytic hydrogenation of alkynes. Step-by-step, hydrogenation creates an alkene first, which is then further hydrogenated to create an alkane.
How are alkenes created?
Elimination processes, which remove two atoms from adjacent carbon atoms to create a double bond, are typically used to create alkenes. Dehydration of alcohols, dehydrohalogenation of alkyl halides, and dehalogenation of alkanes are all steps in the preparation process.
What is an alkyne's end product?
Similar to alkenes, the major reaction route for alkynes is "addition," which entails rupturing the C-C bond and creating two new single bonds to carbon. Alkenes are the end product of addition reactions to alkynes, and as we just saw, addition reactions also occur in alkene reactions.
To know more about Alkynes visit;
https://brainly.com/question/23508203
#SPJ4
A sample of gas weighs 3.33 g and occupies a volume of 1.365 l at 95 °c and 790 torr. identify the gas sample.
Answers
The gas sample is having molar mass of 64 g/mol
Identify the gas sample ?The question is incomplete, here is the complete question:
A sample of gas weighs 10.1 g and occupies a volume of 5.65 L at 60 °C and 580 torr. Identify the gas sample.
(a) (molar mass = 119.4 g/mol)
(b) (molar mass = 44.02 g/mol)
(c) (molar mass = 64.07 g/mol)
(d) (molar mass = 17.03 g/mol)
(e) (molar mass = 70.90 g/mol)
To calculate the molar mass of the gas, we use the equation given by ideal gas equation:
PV = nRT
P = Pressure of the gas = 580 torr
V = Volume of the gas = 5.65 L
w = Weight of the gas = 10.1 g
M = Molar mass of gas = ?
R = Gas constant =62.36 L
T = Temperature of the gas = 60 degree C = ( 60+ 273) K = 333K
Putting values in above equation, we get:
580 torr * 5.65 l = 10.1/ M* 62.364 L
M = 64g /mol
Hence, the gas sample is having molar mass of 64 g/mol
To learn more about gas sample refer
https://brainly.com/question/25736513
#SPJ4
which of the compounds would most readily react with a strong base, such as nanh2, to form a carbanion?
Answers
NaNH2 is a strong base & excellent nucleophile. Benzene diazonium chloride when reacts with hypo phosphorus acid produces to form a carbanion.
What is base?
There are three common definitions of the term base in chemistry known as Arrhenius base, Bronsted base and Lewis base. All definitions are first given by G.-F. Ruel in the mid-eighteenth century.
Therefore, NaNH2 is a strong base & excellent nucleophile. Benzene diazonium chloride when reacts with hypo phosphorus acid produces to form a carbanion.
To learn more about base refer the given link:-
https://brainly.com/question/28907871
#SPJ4
The compound SrBr₂ contains how many atoms?
02
04
01
0 3
Answers
One Strontium atom and two Bromine atoms
Hope this helps. Please give brainliest
Is water an element
Answers
Answer:yes
Explanation:the element of the world is water,fire,air,and earth
It contains hydrogen and oxygen atoms and thus is not an element