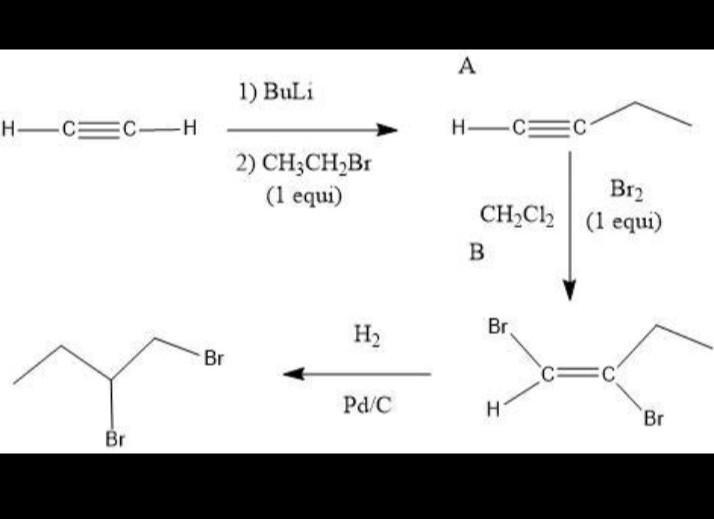
Draw the structures of organic compounds a and b. Indicate stereochemistry where applicable.
Answers
The steric hindrance shown here for butene and 1,2 dibromobutane is cis 2 butene y trans 2 butene.
The better electricity price (and slower response rate) because of the method of large atoms or businesses in a chemical response, as compared to a comparable response related to smaller atoms or businesses.
Steric dilemma is the slowing of chemical reactions because of steric bulk. It is generally manifested in intermolecular reactions, while dialogue of steric outcomes frequently recognition on intramolecular interactions. Steric dilemma is frequently exploited to manipulate selectivity, which include slowing undesirable side-reactions.
Read more about organic;
https://brainly.com/question/1594044
#SPJ4
Related Questions
A colorimeter is an instrument used to measure the amount of _____ absorbed by a solution. This absorbance is proportional to the______of the solute in soli Theat light sound absorbed by a solution. This absorbance is A colorimeter is an instrument used to measure the amount of ______ proportional to the ______of the solute in solution. concentration purity molecular weight
Answers
A colorimeter is a device used to determine the concentration of known solutes present in a solution.
A colorimeter measures the amount of light transmitted through a sample at a user-selected wavelength. You can choose from her four wavelengths:430 nm, 470 nm, 565 nm, 635 nm Features such as automatic sensor recognition and one-step calibration make this sensor easy to use. It is used in chemical and other industries such as color printing, textile manufacturing and paint manufacturing.
A colorimeter measures the intensity of light transmitted through a colored solution and the intensity of light transmitted through the solution. It is based on Lambert's Beer's Law, which states that the absorbance of a substance is directly proportional to its concentration.
Read more about calorimeter;
https://brainly.com/question/15479803
#SPJ4
calculate the mass percent of hoch2ch2oh in a solution made by dissolving 3.2 g of hoch2ch2oh in 43.5g of water.
Answers
TheThe mass percent of HOCH2CH2OH is 6.85%
Mass of solute (ethylene glycol) = 3.2 g
Mass of solvent (water) = 43.5 g
Mass of solution = Mass of solute + Mass of solvent = 3.2 + 43.5 = 46.7 g.
Mass%= mass of solute×100/mass of solution
Mass%=3.2×100/43.5 = 6.85%
What is the relationship between solute and solvent?
A solute is a substance this is dissolved in a solution. The quantity of solvent in a fluid solution is extra than the amount of solute. The solvent is the fabric that commonly comes to a decision the solution’s bodily nation (stable, liquid or gasoline). A solution of liquid and water, for example, has water because the solvent and salt because the solute. Water is also known as the “prevalent solvent” because it can dissolve almost any material better than some other liquid.To know more about solute and solvent, click the link given below:
httpshttps://brainly.com/question/16122098
#SPJ4
ibuprofen, C13H18O2, is the active ingredient in many non-prescription pain relievers.
a) If the tables in a bottle contain a total of 33 g of ibuprofen, how many moles of ibuprofen are in the bottle?
b) How many molecules of ibuprofen are in the bottle?
Answers
Mole measure the number of elementary entities of a given substance that are present in a given sample. Therefore, the moles and number of molecules of ibuprofen is 0.15moles and 9.03×10²²molecules respectively.
What is mole?The SI unit of amount of substance in chemistry is mole. The mole is used to measure the quantity or amount of substance. We know one mole of any element contains 6.022×10²³ atoms which is also called Avogadro number.
Mathematically,
mole =given mass ÷ molar mass
given mass of ibuprofen= 33 g
Molar mass of ibuprofen= 206.29 g/mol
mole of ibuprofen=33 g ÷ 206.29 g/mol
mole of ibuprofen=0.15moles
number of atoms/molecules=number of moles × 6.022×10²³(Avogadro number)
number of atoms/molecules of ibuprofen= 0.15× 6.022×10²³
=9.03×10²²molecules
Therefore, the moles and number of molecules of ibuprofen is 0.15moles and 9.03×10²²molecules respectively.
To know more about mole, here:
https://brainly.com/question/15209553
#SPJ1
Select all the statements that correctly describe what happens when a gas is heated in a flexible container at constant pressure.
a. The gas increases in volume because the particles increase in size.
b. The gas particles collide with the walls of the container with a greater force.
c. The surface area of the walls of the container decreases.
d. The volume of the gas container increases.
e. The gas particles move faster.
Answers
"The gas particles move faster", is the correct statement for a gas when it is heated in a flexible container at constant pressure.
The gas particles in the container collides with the walls of the container with a greater force. Due to this collision the volume of the gas container increases.
Properties of gas molecules
Gas molecules have neither definite shape nor definite volume. They expand to the size of their container.
Gas molecules are fluid, and flow easily inside or outside the container.
Gas molecules have low density, unless compressed.
Gas molecules diffuse (mix and spread out) and effuse (travel through small holes).
Learn more about gas molecules from the link given below.
https://brainly.com/question/16075802
#SPJ4
what kind of intermolecular forces act between a carbon monoxide molecule and a hydrogen iodide molecule?
Answers
Dispersion force act between a carbon dioxide molecule and a hydrogen iodide molecule.
Intermolecular interactions known as London dispersion forces typically act between atoms and molecules that are electrically symmetric, meaning that the electrons are evenly distributed around the nucleus. The van der Waals forces include them. The LDF bears Fritz London's name, a German physicist.
The least powerful intermolecular force is the London dispersion force. When the electrons in two nearby atoms occupy positions that cause the atoms to temporarily form dipoles, the consequence is the London dispersion force, a transient attractive force. The term "induced dipole-induced dipole attraction" is frequently used to describe this effect.
For more information on dispersion force kindly visit to
https://brainly.com/question/7290121
#SPJ4
calculate the percentage by mass of acetic acid in vinegar if it takes 24.5 ml of 0.299 m naoh to neutralize 8.55 grams of vinegar
Answers
The percentage by mass of acetic acid is 5.14%
What is percentage by mass?
It is defined as ratio of mass of substance to the mass of all substance multiply by 100.It is way to express concentration.
CH3COOH+NaOH= CH3COONa+H2O
we have 1 mole of acid for 1mole of base
Now , C=n/V
C= 0.299M ( its M not m so correct question)
V= 24.5 mL
no of moles = 0.299moles/L × 0.0245 L = 0.00733 moles
for neutralization of 0.00733 moles of Noah require 0.00733 moles of CH3COOH
given mass of acetic acid = no. of moles × molar mass
0.00733×60g/mole = 0.4398g
% mass of acetic acid =[ 0.4398g/8.55g]×100
⇒ 5.14%
For more information about mass percentage please visit:
https://brainly.com/question/27574467
#SPJ4
complete the overall reaction catalyzed by the pyruvate dehydrogenase complex. move the compounds and cofactors to the correct answer blanks. two terms will not be used.
Answers
The complete overall reaction is represented below:
Pyruvate +Co-A+NAD+ → Acetyl Co-A+ NADH+CO2
ATP,ADP are 2 components that are not used.
This reaction is catalyzed by pyruvate dehydrogenase. This enzyme binds with the p and breaks some bonds as well as make some bonds to produce final products. After the conversion of pyruvate to the acetyl Co-A, this compound enters into mitochondria and enter into Krebs cycle to make energy. The pyruvate dehydrogenase complex (PDC)three catalyzes the oxidative decarboxylation of pyruvate with the formation of acetyl-CoA, CO2 and NADH (H+) (1,–three). The PDC occupies a key role withinside the oxidation of glucose via way of means of linking the glycolytic pathway to the oxidative pathway of the tricarboxylic acid cycle.
To learn more about pyruvate check the link below:
https://brainly.com/question/16346028
#SPJ4
complete the electron‑pushing mechanism for the reaction by drawing the necessary organic structures and curved arrows for each step. make sure to include all nonbonding electron pairs.
Answers
The process by which water is added to an aldehyde when a base is present, Being electrophilic makes this carbon atom vulnerable to nucleophilic attack; in contrast, oxygen is more electronegative.
What is the definition of electronegativity?An atom's or functional group's propensity to draw electrons to itself is known as electronegativity, which is a chemical property. An atom's electronegativity is influenced by both its number of atoms and the separation of its valence electrons from its charged nucleus.
What is a high electronegative indicative of?The capacity of an atom in a covalent connection to pull shared electrons is known as electronegativity. The value of an element's electronegativity determines how strongly it will attract electron pairs.
To know more about electronegative visit:
https://brainly.com/question/17762711
#SPJ4
sarah used 2.5 cups of cheese in a dish that serves 10 people. what constant of proportionality relates the number of servings to cups of cheese?
Answers
The proportionality constant that links the quantity of servings to cups of cheese is 0.25.
In a recipe that serves 10, Sarah used 2.5 cups of cheese, which is equivalent to:
Let c represent the number of cheese cups: c = 2.5 cheese cups
Let n represent the total number of clients served.
The equation is then expressed as follows:
c ∝ n
then we can introduce the sign for the proportionality constant as;
So, c = kn
then we may enter the given numbers as follows into the equation:
=> 2.5=k (10) (10)
The constant's value can be calculated as follows:
= k=2.5/10
= 0.25
Therefore, 0.25 is the constant of proportionality that links the quantity of servings to cups of cheese.
To know more about moles, visit:
https://brainly.com/question/26416088
#SPJ4
what mass of methanol, ch3oh, must be dissolved in 313.0 ml of ethanol to prepare a 0.750 m solution? the density of ethanol is 0.7893 g/ml.
Answers
7,493.1 g of methanol must be dissolved in 313.0 mL of ethanol to prepare a 0.750 M solution.
What is dissolved?
Dissolved is a term used to describe when a solid material has been completely broken down into particles small enough to be suspended in a liquid. This process is known as dissolution, and can be achieved through a variety of different methods. One of the most commonly used methods is mechanical agitation, which involves using mechanical force such as stirring or shaking to cause the solid particles to break down and become suspended in the liquid. This process can be used to dissolve a wide range of different compounds such as salts, sugars, and acids. Other methods of dissolution include chemical reactions, heating, and the use of solvents. Dissolution is a key part of many chemical processes, and its importance in chemistry cannot be overstated.
Molar mass of methanol (CH3OH): 32.04 g/mol
Molarity of solution: 0.750 mol/L
Volume of solution: 313.0 mL
Therefore, the number of moles of methanol required in solution = 0.750 mol/L × 313.0 mL = 234.75 moles
Mass of methanol required = 234.75 moles × 32.04 g/mol = 7,493.1 g
Therefore, 7,493.1 g of methanol must be dissolved in 313.0 mL of ethanol to prepare a 0.750 M solution.
To learn more about dissolved
https://brainly.com/question/25326161
#SPJ4
Is a mole of gas always 22.4 L?
Answers
Yes, At Standard Temperature and Pressure (STP), 1 mole of any gas will occupy a volume of 22.4 L.
At STP, one mole (6.02×1023 representative particles) of any gas occupies a volume of 22.4L, A mole of any gas occupies 22.4L at standard temperature and pressure (0oC and 1atm).
At STP:
The standard temperature is equal to: 273.15 K = 0°C = 32°F
The standard pressure is equal to: 1 atm = 760 Torr = 760 mm Hg = 101.35 kPa.
1 mol of ideal gas in these conditions has a volume of 22.4 Liters.
The question is incomplete, complete question is
Does 1 mole of gas always occupy 22.4 liters?
To know more about Ideal gas here
https://brainly.com/question/17136449
#SPJ4
What is the chemical formula for CH2O?
Answers
The chemical formula of formaldehyde is CH₂O.
Formaldehyde is a compound that is used in our daily use products like paint coatings and paper based products.
The chemical formula formaldehyde is CH₂O it contains 1 carbon atom one oxygen atom and two hydrogen atom the hydrogen atom and the oxygen atom are connected to the carbon atom by single bond and double bond.
Both the hydrogen atom and the oxygen atom forms are covalent bond between carbon and themselves.
Formaldehyde is a very important and useful compound.
To know more about formaldehyde, visit,
https://brainly.com/question/29550668
#SPJ4
Suppose we completely stopped burning fossil fuels immediately. How many years would it take to return to atmospheric co2 levels from the year 1800, about 600 gtc?.
Answers
Observations show that it may take 50 years for atmospheric CO₂ levels to decline from the 1800s.
Soils and terrestrial plants do not increase the amount of CO₂ in ecosystems every year. Coal may also be the answer.
How much CO₂?Since 2000, global atmospheric carbon dioxide has increased by 43.5 ppm due to use. This is an increase of 12%. Today's record of atmospheric carbon dioxide began with observations recorded at the Mauna Loa Observatory in Hawaii.
Note that atmospheric CO₂ is constantly increasing, contributing to global warming. Recent studies show an average increase of about 50% between 1750 and 1800.
Learn more about atmospheric CO₂ from
brainly.com/question/431949
#SPJ4
What is the Lewis structure for CH2O?
Answers
The Lewis structure of any compound is the representation of the valence electron shared by the atoms in the form of dots beside the chemical symbol of the atom.
The Lewis structure of formaldehyde is given below.
It has one carbon atom, one oxygen atom and two hydrogen atoms.
All the atom are making covalent bond with the central carbon atom.
If we observe in the Lewis structure, We can see that on sharing the electrons with the adjustment items carbon is able to achieve a stable configuration.
As we can see there is one electron pair in sharing with the two hydrogen atom but there are two electron payers in sharing with the carbon atom.
This is the thing that is intended to be shown by a Lewis structure.
To know more about formaldehyde, visit,
https://brainly.com/question/20300458
#SPJ4
what can be said about the concentrations of reactants relative to the concentrations of products at equilibrium?
Answers
At the state of equilibrium of the reaction, the concentrations of reactants relative to the concentrations of products will be as follows [Reactants] = [Products].
In the field of chemistry, equilibrium is described as a state during which the amount of reactants is equal to the amount of product that is being produced from the reaction.
In other words there are equal number of moles of the reactants as well as equal number of moles of the products at the stage of equilibrium for a reaction.
While writing the state of equilibrium in a reaction, we will represent this stage as [Reactants] = [Products]. After this stage of the reaction, more of the reactants will be utilized for the formation of products.
Learn more about equilibrium from the link given below.
https://brainly.com/question/29816521
#SPJ4
100.0 ml of a 0.50 m aqueous nh3 solution is mixed with 200.0 ml of 0.25 m aqueous hi. at 25 ºc the mixture will have a ph of
Answers
100.0 mL of a 0.50 M aqueous NH3 solution is mixed with 200.0 mL of 0.25 M aqueous HI. At 25 ºC the mixture will have a pH of <7
If the choices for pH are 7, greater than 7 or less than 7, then the answer would be less than 7 since this is a titration of a weak base (NH3) with a strong acid HI. And since the moles of NH3 = moles HI, you are at the equivalence point, so the pH will be acidic.
What is titration?
Titration, a process of chemical analysis in which the amount of a sample component is determined by adding to the measured sample a precisely known amount of another substance with which the desired component reacts in a certain, known ratio.To know more about titration, click the link given below:
https://brainly.com/question/13307013
#SPJ4
how many electrons in a ground state argon atom have a magnetic quantum number (ml) equal to -2? g
Answers
The magnetic quantum range or ml describes the orientation in area of a specific orbital. It is SP2 hybridisation for Argon and the no. of electrons are 2.
The feasible values of ml may be decided in view that it is able to handiest be an integer withinside the range l±1. If ml is 0 then the price of l can handiest be same to 0. If the price of the azimuthal quantum range is 0, this corresponds to the s orbital.
Thus, you handiest want to matter the electrons which might be withinside the s orbitals of the argon atom. The electron configuration of Argon is given below. There are 6 electrons which have ml=0 in view that there are 6 electrons in s orbitals (1s, 2s and 3s)
Ar:1s22s22p63s23p6.
Read more about the atom:
https://brainly.com/question/860094
#SPJ4
What happens when H2SO4 and NaOH combine?
Answers
Sodium hydroxide reacts with sulphuric acid and has chemical formula is Naoh+h2so4 producing the product of (NaSO4+H2O) Sodium sulphate and water. This is an acid-base reaction and the product obtained is in the form of salt and water. Sodium sulphate is salt(product of neutralisation reaction).
Sodium hydroxide is an inorganic and strong base which is also known as caustic soda. The chemical formula of sodium hydroxide is NaOH and Sulphuric acid is strong acid with chemical formula H2So4.
When Both H2So4 and NaOH combine, the acid-base reaction will occur. Sodium hydroxide is a base that reacts with an acid, i.e., sulfuric acid to give respective salt and water as the product where Sodium sulphate is salt as a product . The reaction is an example of a neutralisation reaction.
To know more about Neutralisation reaction visit here ; https://brainly.com/question/23008798?referrer=searchResults
#SPJ4
Of the following, circle the substance that is not like the others and explain why.
CO₂
H₂O
SiO₂
He
Answers
Explanation: He is the symbol for Helium. Since Helium is an element it is classified as a pure substance. All of the rest of these choices are classified as compounds.
Brainliest is appreciated!
A structure containing a central atom surrounded by three electron groups will have a _____________planar arrangement in which the ideal bond angle is_______________ °.
Answers
A structure containing a central atom surrounded by three electron groups will have a Trigonal planar arrangement in which the ideal bond angle is 120 °.
What is Central Atom?
The ligands in coordination chemistry, as was previously mentioned, either have a single pair of electrons or a negative charge. As a result, they have an excess of electron density that can be donated to the metal's open orbital. The centre atom is the metal atom to which the ligands bond. The central ion is the metal ion to which the ligands attach. As an illustration, the core ion in K3[Fe(CN)6 is Fe3+. These core atoms and central ions serve as Lewis acids, or acceptors of the electron pair.
As d-block elements function as central atoms and central ions in coordination complexes, we must thoroughly comprehend transition metals in order to comprehend this.
Learn more about Central Atom from given link
https://brainly.com/question/29078422
#SPJ4
Calculate the mass of oxygen needed to react 10g of calcium to form calcium oxide.
Answers
The mass of oxygen needed to react 10.0g of calcium to form calcium oxide is 4 g.
The data given in the question is;
mass of the calcium = 10 g
As we know that
atomic mass of oxygen = 16 g/mol
atomic mass of calcium = 40 g/mol
The given chemical reaction;
2Ca + O2 → 2 CaO
from the reaction above we can deduce the following;
80 g of calcium : 32 g of oxygen
10 g of calcium : ?
10 × 32 / 80 = 4g
Thus, the mass of oxygen needed to react 10.0g of calcium to form calcium oxide is 4 g.
To look more about calcium oxide click here
brainly.com/question/20417380
#SPJ4
Which solution of salt nacl or CaCl2 is better to salt the roads?
Answers
Calcium chloride is a better solution of salt to salt the roads.
In the countries where the climate is very cold, the solution of calcium chloride and water is mixed and used for clearing the ice on the road.
The mixture of calcium chloride CaCl₂ and water is called eutectic mixture.
This mixture has a very low freezing point in comparison to sodium chloride.
Sodium chloride NaCl, is also a good substitute for clearing ice but it has a much higher freezing point in comparison to calcium chloride.
This is why calcium chloride is always preferred over sodium chloride because it becomes easier for us to clean the roads.
To know more about calcium chloride, visit,
https://brainly.com/question/12216405
#SPJ4
What does it mean to be in transition?
Answers
Transition means change of one phase of matter into another.
Matter is interconvertible in nature. There are three phases of transition: Melting, freezing and evaporation.
The process of conversion of solid form of matter into liquid form is known as melting. Examples of melting are such as: melting of ice, melting of wax etc.
The process of conversion of liquid form of matter into solid form is known as freezing. Formation of ice is an example of freezing.
And, the process of conversion of liquid form of matter into gaseous form is known as evaporation. Boiling of water is an example of evaporation.
To know more about transition here
https://brainly.com/question/17283694
#SPJ4
What are the effects of transition?
Answers
The effects of transition are: absorption of energy and release of energy
Transition of matter is defined as the conversion of matter from one phase to another. The three states of matter i.e solid, liquid and gas are interconvertible in nature.
Melting, freezing and evaporation are the three phases of transition. And these transition occurs as a result of absorption of energy and release in energy, which also results in the change of shape of the matter.
Melting of ice takes place by absorption of energy. Freezing of water into ice takes place by releasing energy and on evaporation also, energy is released.
To know more about effects of transition here
https://brainly.com/question/19983755
#SPJ4
When solid sodium hydroxide dissolves in water, the δh for the solution process is −44.4 kj/mol. if a 13.9 g sample of naoh dissolves in 250.0 g of water in a coffee-cup calorimeter initially at 23.0 °c. what is the final temperature of the solution? assume that the solution has the same specific heat as liquid water, i.e., 4.18 j/g·k.
Answers
When solid sodium hydroxide dissolves in water, δh for solution process is −44.4 kj/mol then final temperature of the solution is 23.758 °C
q=mc(T2-T1)
T2=q/mc+t1
T2= -44/13.9×4.18+23
T2=23.758 °C
Temperature is the unit used to describe heat or cold. It illustrates the natural flow of heat energy from a hotter to a cooler body and can be expressed in terms of a variety of arbitrary scales (one at a lower temperature). An iceberg has a much higher total heat energy than a match, despite the fact that a match burns at a much higher temperature. Temperature and a thermodynamic system's energy are not the same thing. In contrast to extensive features like mass or volume, such as temperature is referred to as an intense property—one that is independent of the amount of material being addressed—along with pressure, density, and other similar qualities.
To know more about temperatures visit : https://brainly.com/question/10576021
#SPJ4
Entropy never decreases in a spontaneous process. give an example to support this statement.
Answers
The transfer of energy by heat from colder bodies to hotter bodies is the perfect example for entropy of a spontaneous reaction.
Spontaneous reactions are described as those chemical or biological reactions that usually take place without the influence of external factors. Non- Spontaneous reactions are described as those chemical reactions that generally requires an energy input to proceed or that cannot take place without the influence of external factors.
The transfer of energy by heat from colder bodies to hotter bodies is an example of spontaneous process in which the entropy of the system of bodies increases. The melting of the ice cube placed in a room causes an increase in the entropy of the room.
Learn more about spontaneous reaction from the link given below.
https://brainly.com/question/29821533
#SPJ4
chemical analysis on a rock specimen has shown a ratio of 1:3 for radioactive parent to stable daughter product. if the radioactive isotope in this rock has a half-life of 10,000 years, how old is this rock?
Answers
This rock is 20,000 years old if the radioactive isotope it contains has a half-life of 10,000 years.
Not all of an element's atoms instantly transform into atoms of another element during natural radioactive decay. There is value in being able to express the rate at which a process occurs since the decay process takes time. Within one half-life, the amount of radioactive nuclei present at any particular time will be reduced to half. The procedure of radioactive dating involves using certain radioactive nuclides to establish an object's approximate age.
Age = nxt1/2 is the formula used to determine an object's age using isotope dating, where n is the quantity of half-lives.
To learn more about radioactive isotope click here https://brainly.com/question/29611802
#SPJ4
The gradual increase in the temperature of the atmosphere is known as_______?
A.Greenhouse effect
B.Greenhouse gases
C.Global warming
D.Carbon dioxide
Answers
Answer:
Global warming
Explanation:
It is known as "Global warming" is a condition wherein the Earth's temperature increases in a gradual manner.
So the answer is "Global warming"
Answer:
C Global
Explanation:
a pure substance which can only be separated into two or more simpler substances using chemical changes is called
Answers
A pure substance that can only be separated into two or more simpler substances using chemical changes is called a chemical compound.
A chemical compound is a type of pure substance that is made up of two or more elements that are chemically combined in a fixed ratio. The elements in a chemical compound are bonded together by chemical bonds, which are the forces that hold the atoms together in a compound. Examples of chemical compounds include water (H2O), table salt (Nacl), and carbon dioxide (CO2). These compounds are made up of two or more elements that are chemically bonded together in a fixed ratio, and they cannot be separated into their constituent elements using physical methods such as filtration or distillation. Instead, chemical reactions are required to separate the elements in a chemical compound.In contrast, a pure substance that can be separated into simpler substances using physical methods without undergoing a chemical change is called a mixture. Examples of mixtures include air and seawater. These substances are made up of two or more substances that are not chemically bonded together, and they can be separated into their constituent substances using physical methods such as filtration or distillation.
To know more about elements-
https://brainly.com/question/14347616
#SPJ4
What happens to the kinetic energy of its molecules as ice melts into water?