Answers
Answer:
1. A. 9 eggs
2. B. 5:3
3. C. Exactly 44 g
4. C. Mass and atoms only
5. D. 6.81 g of PH₃
6. C. 88.4%
7. B. O₂
8. D. 30.8 g CO₂
9. B.
10. B.
11. C. 99 g
12. D. mole ratio
13. C. Theoretical
14. A. 6.0 mol H₂O
15. D. 39.7 g CH₃OH
16. A. 650 g HgO
17. D. 8.8 mol H₂
18. B. 82.6%
Explanation:
1. The number of eggs it takes to make 1 cake = 3 eggs
The number of eggs it takes to make 3 × 1 = 3 cake = 3 × 3 = 9 eggs
Therefore, the correct option is;
A. 9 eggs
2. The given reaction is presented as follows;
C₃H₈(g) + 5O₂(g) → 3CO₂(g) + 4H₂O(l)
In the above reaction, we have;
Moles of , O₂, reacted = 5 moles
Moles of , CO₂, produced = 3 moles
The ratio of the of O₂ reacted to moles of CO₂ produced = 5 moles:3 moles
∴ The ratio of the of O₂ reacted to moles of CO₂ produced = 5:3
The correct option is;
B. 5:3
3. The reaction is presented as follows;
O₂ (g) + C(s) → CO₂ (g)
From the reaction, 1 mole (12 g) of carbon produces 1 mole of CO₂
The molar mass of CO₂ = The mass of 1 mole of CO₂ = 44.01 g/mol
Given that the reaction is completed, the mass of CO₂ produced = The mass of 1 mole of CO₂ ≈ 44 g
The correct option is;
C. Exactly 44 g
4. The given reaction is presented as follows;
N₂ + 3 F₂ → 2NF₃
The initial number of atom = 2 + 6 = 8
The final number of atom = 2 × 4 = 8
∴ The initial number of atom = The final number of atom
Therefore, the number of atoms is conserved;
The mass of the reactants ≈ 28 g/mol + 3 × 37.996806 g/mol ≈ 141.993612 g/mol ≈ 142 g/mol
The mass of the product ≈ 2 × 71 g.mol = 142 g/mol
∴ The mass is conserved
Moles of reactants = 1 + 3 = 4
Moles of products = 2
∴ The number of moles is not conserved
The correct option is
C. Mass and atoms only
5. The molar mass of P₄ = 123.895048 g/mol
One mole of P₄ (123.895048 g) produces four moles (4 × 34.00) of PH₃
6.20 g of P₄. will produce (4 × 34.00)/(123.895048) × 6.20 g ≈ 6.80576 g ≈ 6.81 g
The correct option is D. 6.81 g of PH₃
6. The percentage yield = ((The actual yield)/(The ideal yield)) × 100
The actual yield of silver = 38.1 g
The ideal yield of silver = 43.1 g
∴ The percentage yield = ((38.1 g)/(43.1 g)) × 100 = 88.3990719258% ≈ 88.4%
The percentage yield = 88.4%
The correct option is C. 88.4%
7. The given chemical equation is presented as follows;
CS₂ (g) + 3 O₂ (g) → CO₂ (g) + 2 SO₂ (g)
The number of moles in 192 g of O₂ = 192 g/(32 g/mol) = 6 moles
Given that 3 moles of O₂ reacts with 1 mole of CS₂ to produce 1 mole of CO₂ and 2 moles SO₂, therefore 2 × 3 = 6 moles of O₂ will reacts with 2 × 1 = 2 moles of CS₂ to produce 2 moles of CO₂ and 4 moles SO₂
∴ The limiting reactant is;
B. O₂
8. The given chemical equation is presented as follows;
2 C₈H₁₈ (g) + 25 O₂ (g) → 16 CO₂ (g) + 18 H₂O (l)
The number of moles in 10 g of C₈H₁₈, n₁ = (10 g)/(114.26 g/mol)
The number of moles of CO₂ produced, n₂ = (10 g)/(114.26 g/mol) × 16/2 ≈ 0.7 moles
The mass of CO₂ produced, m ≈ 44.01 × n₂ ≈ 44.01 g/mol × 0.7 moles ≈ 30.807 grams ≈ 30.8 grams
The theoretical yield of CO₂ from completely burning 10.0 g of C₈H₁₈ ≈ 30.8 grams of CO₂
The correct option is D. 30.8 g CO₂
9. The correct option is B. The limiting reactants determine the maximum amount of product that can be formed
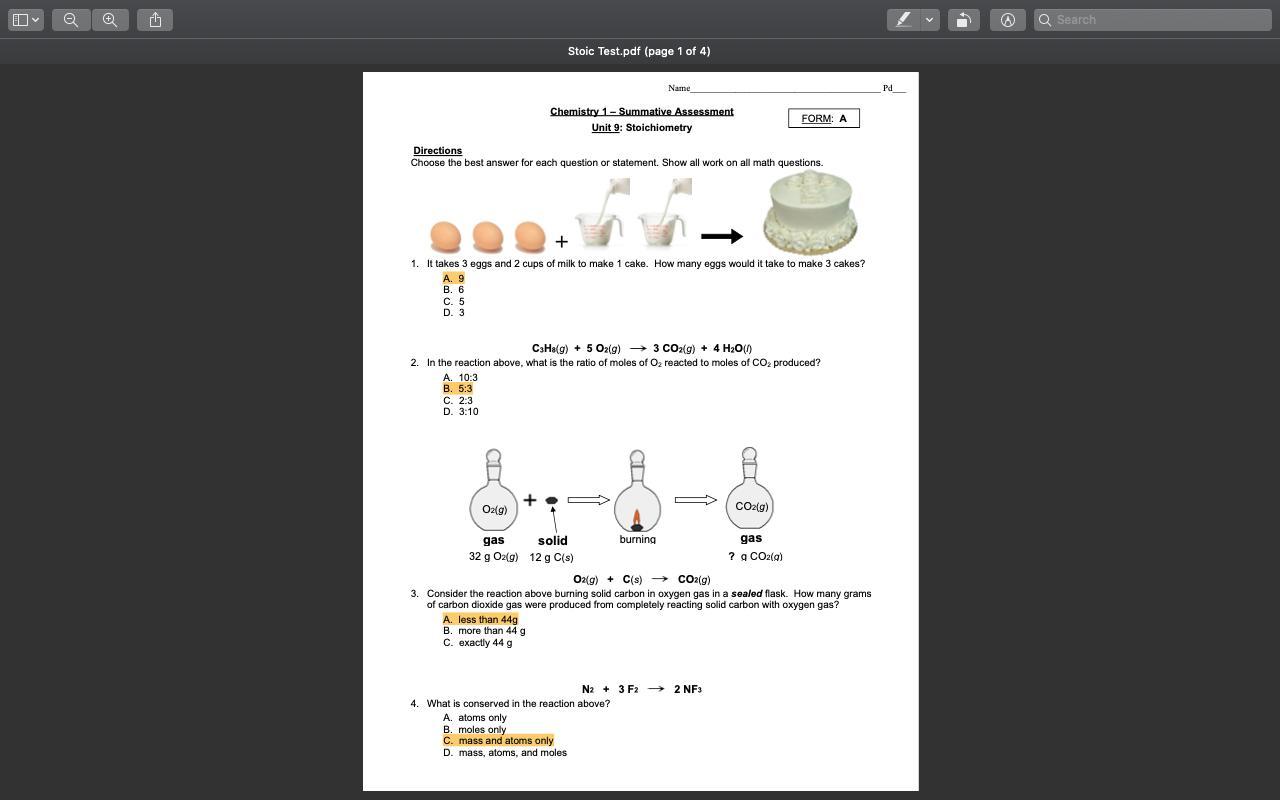
10. Option B, which has 3 atoms of each element combining to form a product with 1 atom of one element and 2 atoms of the other element
The correct option is B.
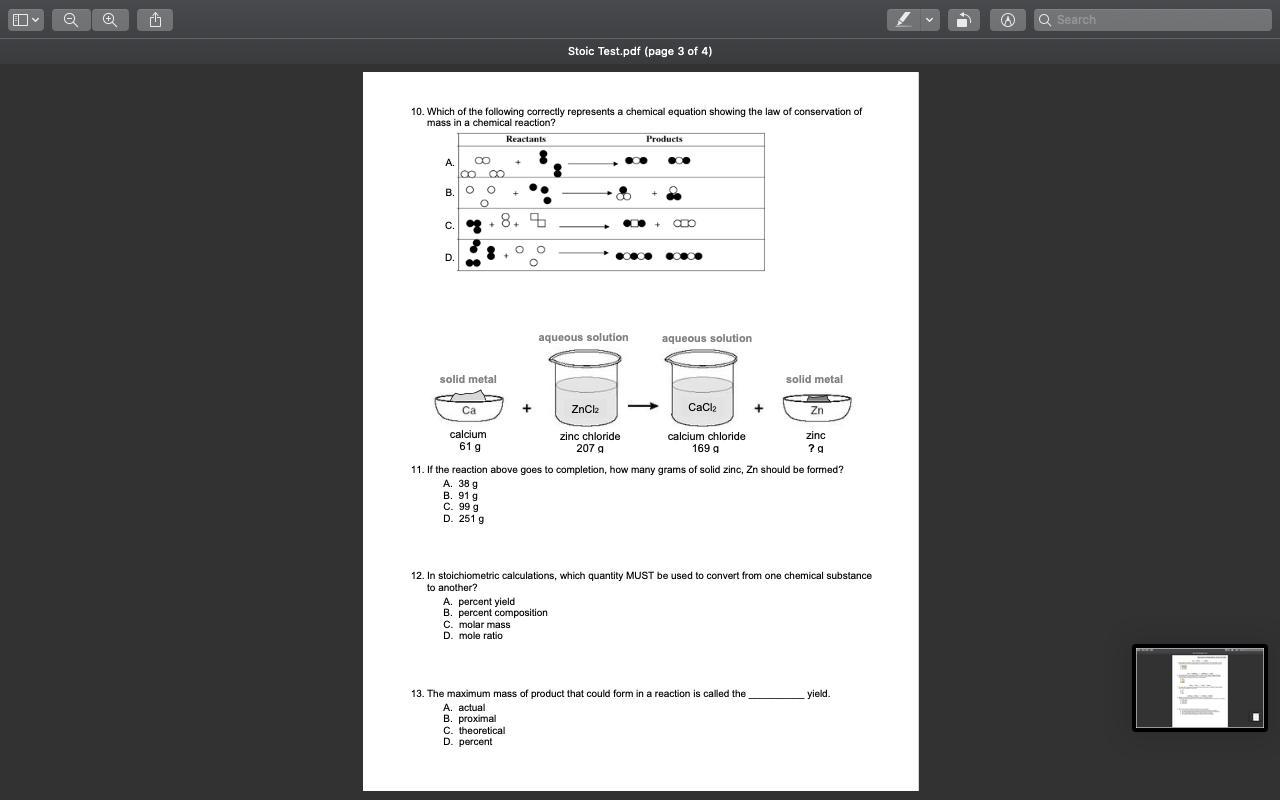
11. By the conservation of mass, we have;
The mass of the reactants = The mass of the products
Let 'x' represent the mass of zinc in the products of the reaction
Therefore, we have;
61 g of calcium + 207 g of zinc chloride = 169 g of calcium chloride + x g of Zinc
∴ x g = 61 g + 207 g - 169 g = 99 g
The mass of zinc in the products of the reaction, x g = 99 g
The correct option is;
C. 99 g
12. The quantity that must be used to convert from one chemical substance to another is the mole ratio
The correct option is D. mole ratio
13. The maximum mass of the product that could form in a reaction is called the theoretical yield, which is option C.
The correct option is C. Theoretical
14. 1 mole of O₂ produces 2 moles of water (H₂O), therefore;
3 × 1 = 3.0 moles of O₂ will produce 3 × 2 = 6 moles of H₂O
The correct option is
A. 6.0 mol H₂O
15. 2 mole × 2.02 g/mol = 4.04 g of H₂ (g) produces 32.05 g CH₃OH (l)
∴ 5 g of H₂ (g) will produce 32.05 g × 5/4.04 ≈ 39.6658416 g ≈ 39.7 g of CH₃OH
The correct option is;
D. 39.7 g CH₃OH
16. 2 (2 × 216.59 g = 433.18 g) moles of HgO produces 1 mole of O₂
1.5 mole of O₂ will be produced by 1.5 × 2 = 3 moles (3 × 216.59 g = 649.77 g ≈ 650 g) of HgO
The correct option is A 650 g HgO
17. 3 moles of H₂ produces 2 moles of NH₃
The number of moles of NH₃ in 100 g of NH₃, n = 100 g/(17.04 g/mol) = 5.868544 moles
The number of moles of H₂ that will produce 5.868544 moles of NH₃ = 3/2 × 5.868544 moles = 8.802816 moles ≈ 8.8 moles
Therefore, the correct option is;
D. 8.8 mol H₂
18. The theoretical yield of PbO = (223.2/331.2) × 9.90 g = 6.67173913 g
The percentage yield = (5.51 g)/(6.67173913 g) × 100 ≈ 82.6%
The correct option is option B 82.6%.
Related Questions
PLS HELP ASAP I WILL GIVE BRAINLYY
Answers
PLS HELP URGENT
Electron dot diagrams
Use your periodic table to write the electron dot diagrams for the following atoms.
1. Calcium (Ca)
2. Polonium (Po)
3. Moscovium (Mc)
4. Boron (B)
5. Fluorine (F)
Answers
PLZ HELP WILL BRAINLIEST balance the following equation: N2 + H2 --> NH3
Write the coefficients that you decide to balance the equation like this 3, 4, 3. If you do not add a coefficient in front of an element or compound, use a 1 in your answer. For instance the for this balanced equation: 2H2 + O2 --> 2H2O you would write your answer: 2, 1, 2
Answers
Answer:
1,3,2
Explanation:
N2 + 3H2-----> 2NH3
(Science) Help Please!
Answers
Answer:
product
Explanation:
It is the right answer.
"Don't use such phrases here, not cool! It hurts our feelings :( "
IS THE COMMENT EVERY DANG TIME YOU TRY TO TYPE ANYTHING TO POST! anyone else having this problem?
Answers
Answer:
ye, i always have that problem. my stuff gets deleted too :'((
The cells of all organisms must produce energy in order for the cell to survive and function. The diagrams below show
different parts of an animal cell and a plant cell.
In which organelle is energy produced in both plant and animal cells?
O Ribosomes
Nucleus
Chloroplast
O Mitochondria
Answers
Answer:
Mitochondria Is produced by both animal and plant cells
Explanation:
A compound and oxidant react to produce heat and a new product is what reaction
Answers
Answer:
A compound and oxidant react to produce heat and new product is chemical reaction.
What is the main function of the cell membrane in an animal cell?
Answers
Answer:
A cell's plasma membrane, also known as the cell membrane, provides protection. It also maintains a constant atmosphere within the cell, and the membrane serves a variety of purposes. The first is to transport nutrients into the cell, and the second is to transport toxic compounds out
Explanation:
Please help me, I am confused
Answers
Answer:
B
Explanation:
Ionic compound can conduct electricity
Please help!!! What is electrolysis?
Answers
Explanation:
plzz tell me the ans even i also want to know plzzzz someone say ans
Answer:
electrolysis is a procedure that uses direct electric current to achieve an otherwise non-spontaneous chemical reaction and is important in the separation of elements from naturally occurring
What do properties of matter tell us about a substance?
Answers
Answer:
Chemical properties of matter describes its "potential" to undergo some chemical change or reaction by virtue of its composition. What elements, electrons, and bonding are present to give the potential for chemical change. It is quite difficult to define a chemical property without using the word "change"
Explanation:
How to convert celcius to Kelvin?
Answers
Answer:
temperature
Explanation:
Calculate the number of moles of NaOH contained in 250. mL of a 0.05M solution?
Answers
Answer:
0.0125 moles of NaOH are present
Explanation:
Molarity, M, is an unit of concentration widely used in chemistry. Is defined as the ratio between moles of solute (In this case, NaOH) and liters of solution.
250.0mL are = 0.250L of solution. As the molarity of the solution is 0.05M = 0.05moles / 1L, the moles present are:
0.250L * (0.05moles / 1L) =
0.0125 moles of NaOH are present0.32 moles of oxygen gas has a temperature of 27°C and pressure of 2 atm in a closed container. What is the volume?
Answers
Answer:
3.9 L
First convert temperature to Kelvin
then use the ideal gas law
use algebra to solve for V
Since your solving for volume, your answer should be in Liters.
What would happen to a plant that did not perform cellular respiration in the light?
Answers
Answer:
Explanation:
Cellular respiration does not depend on light... Some plants may go into a form of hibernation during winter when the amount of light during the day is less. The reduction of plant growth and activity would reduce the need for the energy produced by cellular respiration.
How many moles are in 281 g of Ca(OH)2?
Answers
Answer:
3.79 moles
Explanation:
To convert moles to gams of a substance we need to find the molar mass of the substance. For Ca(OH)₂ th molar mass is:
1Ca = 40.08g/mol
2O = 2*16g/mol = 32g/mol
2H = 2*1.01g/mol = 2.02g/mol
The molar mass is:
40.08g/mol + 32g/mol + 2.02g/mol = 74.1g/mol
And moles are:
281g * (1mol / 74.1g) =
3.79 molesSedimentary
Weathering
erosion, transport
deposition
Heat and/or
pressure
Igneous
Metamorphic
The
Rock
Cycle
Intrusion or
eruption
Burial and
extreme heat
Magma
Look at the diagram above.
Which sentence BEST describes a process in the rock cycle?
Igneous rocks can become metamorphic and sedimentary rocks.
Metamorphic rocks can form by the weathering process,
Volcanoes can deposit sedimentary rock to form mountains.
O Sedimentary rocks can melt to become metamorphic rocks.
Answers
Answer:
I am confident that it is the last answer but if you can find a more verified answer I would go for that.
How many milliliters of 0.25M H2SO4 can be prepared from 57 mL of a 3.0M solution of H2SO4?
Answers
Answer:
Why ? Because 1 molecule of H2SO4 gives 2 H+ ions per molecule while only one H+ ion is required to neutralize 1 molecule of KOH. So, 1 molecule of H2SO4 can neutralize 2 molecules of KOH. Hence, we would require 525 ml of 0.03 M H2SO4 to neutralize 525 ml of 0.06 M KOH. How will we prepare 525 ml of 0.03 M H2SO4 ?
Explanation:
Now, we have 0.025 M H2SO4 and we do not know how much volume we have.
We will use the standard N1 X V1 = N2 X V2 for this calculation.
N1=0.025 M; V1=unknown; N2=0.03 M and V2=525 ml.
So V1= (0.03 X 525)/(0.025) = 630 ml.
According to the molar concentration, 684 ml of 0.25 M H₂SO₄ can be prepared from 57 mL of a 3.0 M solution of H₂SO₄.
What is molar concentration?Molar concentration is defined as a measure by which concentration of chemical substances present in a solution are determined. It is defined in particular reference to solute concentration in a solution . Most commonly used unit for molar concentration is moles/liter.
The molar concentration depends on change in volume of the solution which is mainly due to thermal expansion. Molar concentration is calculated by the formula, molar concentration=mass/ molar mass ×1/volume of solution in liters.
In terms of moles, it's formula is given as molar concentration= number of moles /volume of solution in liters.In case of 2 solutions,it is calculated as, M₁V₁=M₂V₂ substitution gives V₁=3×57/0.25=684 ml.
Thus, 684 ml of 0.25 M H₂SO₄ can be prepared from 57 mL of a 3.0 M solution of H₂SO₄.
Learn more about molar concentration,here:
https://brainly.com/question/15532279
#SPJ3
13. If a chemist has 12.3 moles of N H 03, what is the mass of the sample?
Answers
Answer:
209.4831 g
Explanation:
number of moles × molar mass = mass of substance in g
12.3 × ( 14.0067 + 1.00484 × 3 ) = 209.4831 g
100 point and brainlist answer the question below
Answers
Answer:
a
Explanation:
because am pro
Molar Volume of a gas at STP=22.4 L Example 3: Determine the volume of Carbon dioxide created when 15.0 grams of NaHCO3 are decomposed into water, Sodium carbonate, and Carbon dioxide at STP?
Answers
Answer:
20009 is answer is right 1345678899444
Explanation:
thhjbfthvcdthvctyhgffdy
gvhjk ghkde ghjcddxxb hhj hhgddxb ggjbcxdss ggbbsrtyg fy gbsdgh
When magnesium reacts with sulfuric acid the products are magnesium sulfate and hydrogen. If there are 15 grams of magnesium at the start of the reaction, how much magnesium will be present in the magnesium sulfate
Answers
Answer: 15 grams of magnesium will be present in the magnesium sulfate
Explanation:
According to the law of conservation of mass, mass can neither be created nor be destroyed. Thus the mass of products has to be equal to the mass of reactants. The number of atoms of each element has to be same on reactant and product side.
The balanced chemical reaction is:
[tex]Mg+H_2SO_4\rightarrow MgSO_4+H_2[/tex]
As the mass remains conserved at the end of the reaction, there will be 15 grams of Magnesium in magnesium sulphate.
Four major bahiagrass varieties
Answers
what fault block that is located below the fault
Answers
Answer:
it is called the foot block1 point
When substances that make up baking powder are mixed with water,
carbon dioxide gas is formed. This is an example of *
a nuclear reaction
a physical reaction
a change of state
a chemical reaction
Answers
What element is steel mainly composed of?
A. Iron
B. Carbon
C. Manganese
D. Silver
Answers
Hope this helps :)
El acero esta compuesto por Hierro y Carbono
If the mass of all the reactants in a chemical reaction is 100g, what will the mass of all products be?
Answers
3) A child has a toy balloon with a volume of 1.8 liters. The temperature of the balloon when it was filled was 22° C and the pressure was 1.0 atm. If the child were to let go of the balloon and it rose into the sky where the pressure is 0.86 atm and the temperature is 8° C, what would the new volume of the balloon be?
Answers
This is a combined gas law problem, according to which
[tex]\frac{P_1V_1}{T_1} = \frac{P_2V_2}{T_2}[/tex]
where P is the pressure of the gas, V is the volume of the gas, and T is the temperature of the gas, and the subscripts 1 and 2 correspond to the initial and final conditions of the gas. In this problem, we are given the initial pressure, volume, and temperature of the gas in the balloon:
P₁ = 1.0 atm
V₁ = 1.8 L
T₁ = 295.15 K (K = °C + 273.15).
Moreover, we are given the final pressure and temperature of the gas in the balloon.
P₂ = 0.86 atm
T₂ = 281.15 K.
What we want to find is the final volume, V₂, which we can obtain by rearranging the combined gas equation to solve for V₂:
[tex]V_2=\frac{P_1V_1T_2}{T_1P_2} = \frac{(1.0 \text{ atm})(1.8 \text{ L})(281.15 \text{ K})}{(295.15 \text{ K})(0.86 \text{ atm})} \\ V_2 = 1.99 \text{ L}[/tex]
This answer has three significant figures. However, the question as written would warrant an answer that comprises one significant figure (as 8 °C has only one sig fig). In that case, the answer would be 2 L. If the answer is to be given to two significant figures, the volume would then be 2.0 L.
1)Change the following temperatures to Kelvin: 100 C and -20 C
2)Change the following temperatures to Celsius: 273 K and 15 K
Answers
Answer:
373 K; 253 K
0°C ; -258°C
Explanation:
In order to change Temperatures to Absolute Value (Kelvin) you shoud do this sum:
T° in C + 273 = T° in K
For question 1:
100°C + 273 = 373K
-20°C + 273 = 253K
For question 2:
T° K - 273 = T° C
273 K - 273 = 0°C
15 k + 273 = -258 °C
Answer:
-67.8
Explanation:
Ripromano
To help ripromano Sorry email won't work on the school computer had to do it this way. Hope this is the right project
Answers
Answer:
Socrates was a Greek philosopher, considered one of the most important, a master of Plato, who called Aristotle a disciple
Explanation: