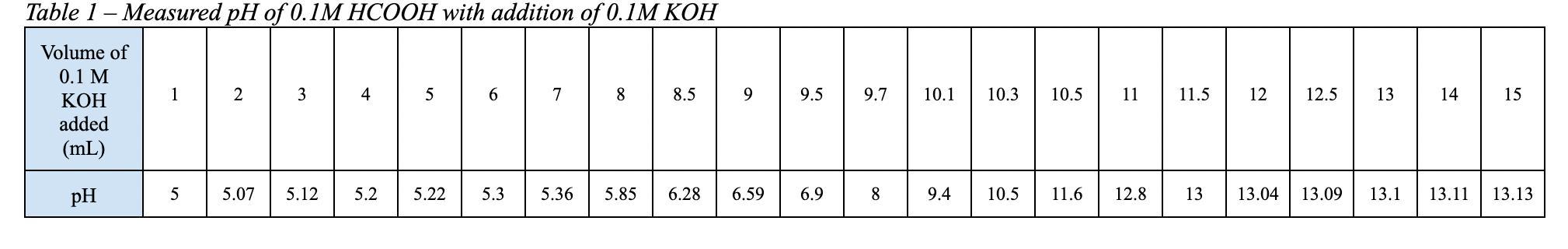
Answers
According to the problem we are adding a 0.1 M solution of KOH to a 0.1 M solution of HCOOH. So, we are titrating a weak acid using a strong base.
The reaction that describes this titration is:
HCOOH (aq) + KOH (aq) <----> HCOOK (aq) + H₂O (l)
The reaction is 1 to 1, since all the coefficients are 1.
The equivalence point occurs when equal number of moles of acid reacts with equal number of moles of base.
Let's find the initial number of moles of metanoic acid that we had:
Initial number of moles of HCOOH = 10.00 mL * 0.1 mmol/mL
Initial number of moles of HCOOH = 1.00 mmol
In the equilibrium point we need the same number of moles of the strong base.
Moles of KOH =
Related Questions
NH3 + O2 → ___NO2 + _H2O
Answers
Answer
Explanation
Given unbalanced equation:
NH₃ + O₂ → ___NO₂ + _H₂O
Step 1: Balance the N atom on both sides by putting 4 as the coefficient of NH₃ and NO₂ on the reactant and product sides of the equation respectively.
4 NH₃ + O₂ → 4 NO₂ + _H₂O
Step 2: Balance the H atom on both sides by putting 4 as the coefficient of NH₃ and NO₂ on the reactant and product sides of the equation respectively.
Calculate the mass percent by volume of 44.1 g of glucose (C₆H₁₂O₆, MM = 180.2 g/mol) in 325 mL of solution.
Answers
13.1% m/v is the mass percent by volume of 44.1 g of glucose (C₆H₁₂O₆, MM = 180.2 g/mol) in 325 mL of solution.
[tex]% m/v=mass solute/volume solution * 100%=42.6g/325mL *100%=13.1%m//v[/tex]% m/v = mass solute/volume solution x 100%
[tex]% m/v=mass solute/volume solution * 100%=42.6g/325mL *100%=13.1%m//v[/tex]% m/v =44.1g/325 mL x 100%
[tex]% m/v=mass solute/volume solution * 100%=42.6g/325mL *100%=13.1%m//v[/tex]% m/v =13.1% m/v
An object's volume in three dimensions is determined by the area encompassed inside its boundaries. It is also known as the object's capability on occasion. Calculating how much is required to fill an object can be done using its volume; for example, how much water is required to fill a bottle, aquarium, or water tank.
The most fundamental and typical kind of three-dimensional form is a sphere. Spheres are frequently seen as balls, globes, holiday lights, oranges, etc.
To know more about volume visit :https://brainly.com/question/24189159
#SPJ1
HCl +H2O -> H3O^+ + Cl^-In the reaction shown above, theis the Bronsted-Lowry base, and theis the Bronsted-Lowry acid.A)Cl;HB)H;ClC)H2O;HClD)HCl;H2O
Answers
H₂O is the Bronsted-Lowry base and HCl is the Bronsted-Lowry acid. Option C is correct.
Explanations:Bronsted-Lowry acid is defined as the ion or molecule that donates a hydrogen ion (H⁺) in a chemical reaction. Since H⁺ is a proton, hence a Bronsted - Lowry acid is a proton donor meaning that we can determine the Bronsted - Lowry acid in a chemical reaction by simply looking out for the molecule that loses a hydrogen ion (proton).
From the chemical reaction given
[tex]HCl+H_2O\to H_3O^++Cl^-[/tex]We can see that hydrochloric acid (HCl) donates a proton to have one less proton on the product side than on the reactant. Therefore, HCl in this case will be the Bronsted-Lowry acid since it acts as the donor.
Similarly, a Brønsted-Lowry base is a molecule or ion that accepts hydrogen ions in a reaction. From the reaction, you can see that water (H₂O) is the molecule that accepts this hydrogen ion to form a hydronium ion (H₃O⁺). Hence, the water molecule will act as a Bronsted - Lowry base
what mass of KHC8H4O4 would be neutralized by 25.25 ml of 0.1034 NaOH
Answers
KHC₈H₄O₄ would be neutralized by 25.25 ml of 0.1034 NaOH then the mass of KHC₈H₄O₄ is 5.151 g
Mass is a measure of the amount of matter in a substance or an object here given data is KHC₈H₄O₄ would be neutralized by 25.25 ml of 0.1034 NaOH
Then the reaction is
KHC₈H₄O₄ (aq) + NaOH (aq) → NaKC₈H₄O₄ (aq) + H₂O
Then the concentration of NaOH is equal to the 0.1 M then we use this and the volume given above to determine the mass of KHC₈H₄O₄
0.1 mol/L NaOH (25.25 ml) ( 1 mol KHC₈H₄O₄/ 1 mol NaOH) (204 g / 1 mol) = 5.151 g KHC₈H₄O₄
5.151 g mass of KHC₈H₄O₄ would be neutralized by 25.25 ml of 0.1034 NaOH
Know more about mass
https://brainly.com/question/4157583
#SPJ1
It took 13,750 Joules of energy to raise the temperature of water to 45.0 °C. If you have 125.0 grams
of water and the specific heat of water is 4.18 J/g °C, what is the starting temperature?
Answers
The initial temperature of water can be calculated from the heat energy using calorimetric equation. The initial temperature is obtained as 18.65 °C.
What is calorimetric equation?Calorimetry is an analytical tool used to determine the heat energy absorbed or released in a chemical reaction. The heat energy q, mass of the substance m and the specific heat capacity c with a temperature difference ΔT is written as follows:
q = m c ΔT.
Here, the heat energy required for raising the temparture of 125 g of waater to 45 °C is 13750 joules . Then, the initial temperature be x, then x can be calculated as follows:
13750 J = 125 g × 4.18 J/g °C × (45 - x °C)
x = 45 - [ 13750 J / (125 g × 4.18 J/g °C) ]
= 18. 65 °C.
Therefore, the initial temperature was 18. 65 °C.
To find more on calorimetry, refer here:
https://brainly.com/question/11477213
#SPJ1
Sodium chloride, NaCL, forms when the metal sodium and the nonmetal chlorine join chemically. What type of substance is sodium chloride?A. a salt compoundB. an elementC. a heterogeneous mixtureD. a solution
Answers
Question 1
Answer & explanation
Sodium chloride is commonly known as table salt. It is an ionic compound with the chemical formula NaCl.
NaCl is a compound
Question 2
Answer & explanation
It is possible to separate a solution by physical means.
What is the energy of a photon of light that has a frequency of 7.87 × 10^15 Hz?
Answers
The question requires us to calculate the energy of a photon given that its frequency is 7.87 × 10^15 Hz.
To calculate the energy of a photon, we can use the following equation:
[tex]E=h\times f[/tex]where h is the Planck's constant (6.626 x 10^-34 Jxs) and f is the frequency (in Hz or 1/s). (Note that the unit Hz is equivalent to s^-1)
Then, applying the frequency provided and the Planck's constant to the equation, we have:
[tex]E=(6.626\times10^{-34}J\mathrm{}s)\times(7.87\times10^{15}s^{-1})=5.21\times10^{-18}J[/tex]Therefore, the energy of the photon is 5.21 x 10^-18 J.
A nurse practitioner prepares an injection of promethazine, an antihistamine used to treat allergic rhinitis. If the stock bottle is labeled 25 mg/mL and theorder is a dose of 15.0 mg, how many milliliters will the nurse draw up the syringe? Express the volume in milliliters to two significant figures.
Answers
Explanation:
We are given: density of promethazine = 25mg/mL
: mass of promethazine = 15.0mg
[tex]\begin{gathered} D\text{ = }\frac{m}{V} \\ \\ \therefore V\text{ = }\frac{m}{D} \\ \\ \text{ = }\frac{15}{25} \\ \\ \text{ = 0.60 mL} \end{gathered}[/tex]Answer:
The nurse will draw up 0.60 mL.
Assign oxidation numbers to each atom in the following compounds or ions: a. HFb. C/4c. H₂0d. Pl3e. CS₂f. Na₂O₂g.H₂CO3h. NO 2i. SO2-4Deforming the oxidation numbers for iron oxide Fe-On (Recall that oxidation numbers are integers)
Answers
Some oxidation number rules
1) The oxidation number of a free element is always 0. The atoms in He and N2, for example, have oxidation numbers of 0.
2) The usual oxidation number of hydrogen is +1.
3 The oxidation number of oxygen in compounds is usually -2. Exceptions include OF2 because F is more electronegative than O, and BaO2, due to the structure of the peroxide ion, which is [O-O]2-.
a) HF, H = +1 F = -1
b)
c) H₂0, H = +1 O = -2
d) Pl3, P = +3 I = -1
e) CS₂, C = +4 S = -2
f) Na₂O₂, Na = +1 O = -1
g) H₂CO3 H = +1 C =+4 O =-2
h) NO2 N = +2 O = -2
i) SO2-4 S =+6 O = -2
Name the following ester molecule:OA. ethyl butanoateB. propyl pentanoateC. methyl heptanoateD. methylpropanoate
Answers
Answer
A. ethyl butanoate
Explanation:
Esters are known for their distinctive odors and are commonly used for food aroma and fragrances. The general formula of an ester is RCOOR'.
Esters are formed through reactions between an acid and an alcohol with the elimination of water.
Esters are named as if the alkyl chain from the alcohol is a substituent. No number is assigned to this alkyl chain. This is followed by the name of the parent chain from the carboxylic acid part of the ester with an –e remove and replaced with the ending –oate.
Hence, the name of the given ester is:
A. ethyl butanoate
How many moles are in 127.49 grams of Ec2H406?Ec has a molar mass of 31.79 grams/mole.(Hint: You need to determine the molar mass of Ec2H406 to solve this problem).
Answers
As the hint says the first thing we have to do is to determine the molar mass of our compound. To do so we need the molar mass of one atom of each element present in our compound.
molar mass of Ec = 31.79 g/mol
molar mass of H = 1.01 g/mol
molar mass of O = 16.00 g/mol
Since one molecule of Ec₂H₄0₆ has 2 atoms of Ec, 4 atoms of H and 6 atoms of O, we can find the molar mass of Ec₂H₄0₆ like this:
molar mass of Ec₂H₄0₆ = 2 * molar mass of Ec + 4 * molar mass of H + 6 * molar mass of O
molar mass of Ec₂H₄0₆ = 2 * 31.79 g/mol + 4 * 1.01 g/mol + 6 * 16.00 g/mol
molar mass of Ec₂H₄0₆ = 163.62 g/mol
Now we can find the number of moles that we have in 127.49 g of Ec₂H₄0₆.
number of moles = mass of sample/molar mass
number of moles = 127.49 g / (163.62 g/mol)
number of moles = 0.7792 moles
Answer: Therea are 0.7792 moles in 127.49 g of our compound.
can anyone help me with this question
Answers
6.5 moles of gas will fill 1.7 litres at 1.3 atm at the temperature (in K). 546atmK273K=P, and 1atm273K = P.
How would you define temperature?Temperature is a measurement used to represent hotness and coolness on any of a number of scales, including Fahrenheit or Celsius. According to temperature, heat energy will naturally move from a hotter (body with a high temp) to a cooler (body with such a lower temperature) (one at a lower temperature).
What portion of your body is the hottest?The scrotum is the area of the body with the highest temperature (37°C), following by the ears, urine, as well as the mouth. The coldest temperature on human body that can usually be measured is the armpit (35.9°C).
To know more about temperature visit:
https://brainly.com/question/19572385
#SPJ13
Ethanol, C,H,O, is added to gasoline to produce "gasohol,"a fuel for automobile engines. How many grams of O₂ arerequired for complete combustion of 421 g of ethanol?2CO₂(g) + 3H₂O(g)C₂H₂OH()+ 30₂(g)Ethanol
Answers
We have the ethanol combustion reaction to produce CO2 and water. The balanced equation of the reaction is:
[tex]4C_2H_2OH+9O_2\rightarrow8CO_2+6H_2O[/tex]We must first find the moles of ethanol corresponding to 421 grams. To do this we divide the mass by the molar mass of ethanol. The molar mass of ethanol is: 46.07g/mol. The moles of ethanol will be:
[tex]molC_2H_2O=421gC_2H_2O\times\frac{1molC_2H_2O}{46.07gC_2H_2O}=9.14molC_2H_2O[/tex]Now, by the stoichiometry of the reaction, we see that the Oxygen to Ethanol ratio is 9/4. So, the moles of oxygen needed will be:
[tex]molO_2=9.14molC_2H_2O\times\frac{9molO_2}{4molC_2H_2O}=20.6molO_2[/tex]We find the grams of oxygen by multiplying the moles by the molar mass. The molar mass of O2 is 31.9988g/mol.
[tex]gO_2=20.6molO_2\times\frac{31.9988gO_2}{1molO_2}=658gO_2[/tex]Answer: To complete the combustion of 421 g of ethanol are needed 658 grams of oxygen
1) Write the equation for the reaction used to generate oxygen gas. Word Equation: Formula Equation:
Answers
For the generation of oxygen gas, hydrogen peroxide is generally used and by means of a catalyst, the peroxide is decomposed into two compounds: Liquid water and oxygen gas. The reaction is as follows:
Word equation
Hydrogen peroxide --->Water + Oxygen
Formula equation
[tex]2H_2O_2(aq)\rightarrow^{}2H_2(l)+O_2(g)[/tex]It is important to note that the reaction needs a catalyst.
A container of an ideal gas is at STP. It holds 2.90mol of gas.What is the volume of the gas?L
Answers
Explanation:
We are given: 2.90mol of ideal gas
At STP: Pressure = 1 atm
: Temperature = 273.15 K
: R = 0.08205 L.atm/mol.K
Using the ideal gas law:
[tex]\begin{gathered} PV\text{ = nRT} \\ \\ \therefore V\text{ = }\frac{nRT}{P}\text{ = }\frac{2.9\text{ }\times0.08205\times273.15}{1}\text{ = 64.99 L} \end{gathered}[/tex]Answer:
V = 64.99 L
What is the total number of moles of products formed when 1.20 moles of ammonia reacts? 4NH3+5O2 --> 4NO+6H2O
Answers
Explanation:
Step 1 - We need to rewrite the equation here:
4NH3+5O2 --> 4NO+6H2O
The ratio between ammonia (NH3) and the products are:
NH3 and NO:
4:4
NH3 and H2O:
6 H2O
So if we have 1.2 moles of ammonia:
1.2 moles of NH3 ---- x moles of NO
4 moles of NH3 ---- 4 moles of NO
x = 1.2 moles of NO
1.2 moles of NH3 --- x moles of H2O
4 moles of NH3 --- 6 moles of H2O
4x = 7.2
x = 7.2/4
x = 1.8 moles of H2O.
Answer: The total number of moles of products formed is 1.2 moles of NO and 1.8 moles of H2O.
A volume of 56.0 mL of He at STP. a. How much weight? b. How many molecules?
Answers
Answer
Number of moles = 0.0025 mol
Step-by-step explanation:
Given that,
The volume of He at STP is 56.0 ml
Part a:
Find the mole
According to the standard conversion
1 mol = 22.4L
Firstly, we need to convert 56.0ml to L
This can be done by dividing by 1000
56.0mL = 0.056L
1 mole = 22.4L
x mole = 0.056L
Cross multiply
1 * 0.056 = x * 22.4
0.056 = 22.4x
Divide both sides by 22.4
x = 0.056 / 22.4
x = 0.0025 mole
Hence, the mole of Helium is 0.0025 moleHe
How many grams of H2O are in 0.2 moles of H2O (molar mass 18.01 g/mol)?
Answers
The number of grams in 0.2 moles of water is 3.602grams.
How to calculate mass?The mass of a substance can be calculated by multiplying the number of moles of the substance by its molar mass as follows:
Mass = molar mass × no. of moles
According to this question, there are 0.2 moles in water. Water has a molar mass of 18.01g/mol. The mass can be calculated as follows:
Mass = 0.2 moles × 18.01g/mol
Mass = 3.602grams.
Therefore, 3.602 grams is the mass of the water molecule.
Learn more about mass at: https://brainly.com/question/16386473
#SPJ1
Sodium reacts with oxygen to produce sodium oxide.4Na(s)+O2(g)→2Na2O(s)If you have 16.0 g of Na , how many grams of O2 are required for the reaction?
Answers
5.57 grams
Explanations:Given the reaction between sodium and oxygen expressed as:
[tex]4Na(s)+O_2(g)\rightarrow2Na_2O(s)[/tex]Given the following parameters
Mass of sodium = 16.0g
Determine the moles of sodium
[tex]\begin{gathered} moles\text{ of Na}=\frac{mass}{molar\text{ mass}} \\ moles\text{ of Na}=\frac{16}{23} \\ moles\text{ of Na}=0.6957moles \end{gathered}[/tex]According to stoichiometry, 4 moles of sodium reacts with 1 mole of oxygen, the moles of oxygen that reacted is given as;
[tex]\begin{gathered} moles\text{ of O}_2=\frac{0.6957}{4} \\ moles\text{ of O}_2=0.1739moles \end{gathered}[/tex]Determine the mass of oxygen that reacted
[tex]\begin{gathered} mass\text{ of O}_2=mole\times molar\text{ mass} \\ mass\text{ of O}_2=0.1739\times32 \\ mass\text{ of O}_2=5.57grams \end{gathered}[/tex]Hence the mass of oxygen that reacted is 5.57 grams
I need help on number 1 please
Answers
Answer:
Atom 1 and Atom 4 of the elements below are in the same group in the periodic table.
(b) Determine the kilocalories needed to heat 2.55 kg water from 21 °C to 37 °C.
Answers
Calculations:
We are given the following parameters :
• Mass = 2.55kg = 2.55*1000= 2550
,• T1 = 21°C
,• T2 = 37°C
,• Specific heat of water = c = 1cal/g°C
We will calculate q (heat ) as follows :
[tex]\begin{gathered} Q\text{ = m }\cdot c\cdot\Delta T \\ \text{ = 255}0\cdot\text{ }1\cdot\text{ ( 37-21)} \\ \text{ =}2550\cdot\text{ 16} \\ \text{ = 40800 calories }\ldots\ldots(\text{divide by 1000 to get to kilicalories) } \\ \text{ =40.8 kilocalories } \end{gathered}[/tex]Pls help me I don’t know how to do this asap help
Answers
Answer:
[tex]17.65\text{ \%}[/tex]Explanation:
Here, we want to get the percentage by mass of the water molecules in the given compound
To get this, we have to divide the molar mass of water by the molar mass of the percentage and write the answer as a percentage
The molar mass of water is 18 g/mol
The molar mass of MgCO3 is 84 g/mol
Thus, the percentage by mass of water will be:
[tex]\frac{18}{18\text{ + 84}}\text{ }\times\text{ 100 \% = 17.65 \%}[/tex]The definition of a limiting reactant can be summarized as:
Answers
Answer:
Explanation:
Here, we want to get the correct answer choice
The limiting reactant is the reactant that gets used up and that dictates the amount of products we can have
Take for example, we want to build a car, and we have 5 steering wheels but 100 tyres
A car needs 1 steering wheel and 4 tyres. Thus, the number of steering wheels will determine the number of possible cars produced
The steering wheel is thus the limiting reactant
The correct answer choice is that the reactant that is usede up is the limiting reactant
Which element ,cesium or francium easily loses electron ? Justify your answer in terms of atomic size and attraction towards the positive nucleus
Answers
Francium easily loses electrons than cesium
Explanations:Both Cesium and Francium are Group 1 elements (Alkali metals). This shows that the elements in this group have one valence electron in their outermost shell and can easily lose this electron to form a stable atom.
According to the element in the group, Lithium has the least tendency to loose its valence electron while Francium has the highest tendency to lose its valence electron because its outermost electron gets further from the positive nucleus and less attracted by the nucleus compared to the Caesium element.
Also, the atomic size of Francium is greater than that of Caesium. This also shows that francium easily loses electrons than cesium
When bond strength increase .number of hydrogen decrease .Why
Answers
Answer:
As bond strength increases, the atoms in the bond are pulled more tightly together.
Explanation:
As the bond energy increases the bond length decreases
GOOD LUCK!
This is the question I need help with. I would really love to understand how reduction occurs here. Thanks in advance for helping me understand this.Which option describes a situation in which reduction occurs?Cl2 becoming Cl–Cl, 2, becoming Cl, –Xe2+ becoming Xe6+Xe, 2+, becoming Xe, 6+Al becoming Al3+Al becoming Al, 3+S2– becoming S
Answers
In chemistry, reduction means any of a class of chemical reactions in which the number of electrons associated with an atom or a group of atoms is increased.
You can say that a compound that is gaining electrons is being reduced.
The situation where the reduction is occurring is:
[tex]Cl^2\to Cl^-,[/tex]When the reduction occurs, the oxidation number (which is the superindex), it seems that is reducing this number. In this case, 2+ become 1-.
Remember: when the oxidation number is reducing, it means that the compound or element is a reduction (gaining electrons) and when the oxidation number is increasing, it means that it is oxidation (losing electrons) such as the other cases of the problem.
If gas has a pressure of 954 atm and temp of 728 k and the temp changes to 951 k what is the new pressure ?
Answers
We have a change of pressure and temperature. To solve and find the new pressure we will assume that the volume and moles of the gas remain constant, therefore we can apply Gay-Lussac's law which tells us:
[tex]\frac{P_1}{T_1}=\frac{P_2}{T_2}[/tex]Where,
P1 is the initial pressure of the gas, 954atm
T1 is the temperature of the gas, 728K
T2 is the final temperature, 951K
P2 is the final pressure, =?
We clear P2 and replace the known data:
[tex]\begin{gathered} P_2=\frac{P_1}{T_1}\times T_2 \\ P_2=\frac{954atm}{728K}\times951K=1246atm \end{gathered}[/tex]Answer: The new pressure will be 1246atm
Please help solve What is the molarity of a solution that has 2.5 mol of NaOH dissolved in 10.0 L of H2O?
Answers
ANSWER
The molarity of the solution is 0.25M
EXPLANATION
Given that;
The mole of NaOH is 2.5 mol
The volume of the solution is 10.0L
Follow the steps below to find the molarity of the solution
The molarity formula is given below as
[tex]\begin{gathered} \text{ Molarity = }\frac{\text{ number of moles }}{\text{ volume of solution}} \\ \end{gathered}[/tex]Substitute the given data into the above formula
[tex]\begin{gathered} \text{ Molarity = }\frac{\text{ 2.5 }}{\text{ 10.0}} \\ \text{ Molarity = 0.25 M} \end{gathered}[/tex]Therefore, the molarity of the solution is 0.25M
What is the limiting reactant if 2.50 moles of zinc react with 1.00 mole of sulfur? Zn + S—-> ZnS
Answers
Step 1
The reaction provided:
Zn + S => ZnS (completed and balanced)
-------------------
Step 2
Information provided:
2.50 moles of Zn
1.00 mole of S
--------------------
Step 3
The limiting reactant limits the amount of product formed.
By stoichiometry, procedure:
Zn + S => ZnS
1 mole Zn ------------- 1 mole S
2.50 moles Zn -------------- X
X = 2.50 moles Zn x 1 mole S/1 mole Zn = 2.50 moles S
For 2.50 moles of Zn, 2.50 moles of S are needed but there is only 1.00 mole of S. Therefore, the limiting reactant is S.
Answer: Sulfur (S)
NaOH(s) was added to 1.0 L of HClO4(aq) 0.43 M. Calculate [H3O+] in the solution after the addition of 0.10 mol of NaOH(s).Calculate [H3O+] in the solution after the addition of 0.81 mol of NaOH(s).
Answers
Answer:
a) 0.33 M
b) 2.63x10^-14 M
Explanation:
1st) It is necessary to write and balance the chemical reaction:
[tex]HClO_4+NaOH\rightarrow NaClO_4+H_2O[/tex]From the balanced reaction we can see that 1 mole of HClO4 reacts with 1 mole of NaOH.
2nd) Since the relation between the acid (HClO4) and the base (NaOH) is 1:1, the 0.10 moles of NaOH will react with 0.10 moles of acid:
[tex]\begin{gathered} RemainingSolution=0.43moles\text{ - }0.10moles \\ RemainingSolution=0.33moles \end{gathered}[/tex]So, in the solution 0.33 moles of HClO4 will remain unreacted, and the concentration of H3O+ will be 0.33M.
3rd) In this case we can proceed the same as in the first part:
[tex]\begin{gathered} RemainingSolution=0.43moles-0.81moles\text{ } \\ RemainingSolution=-0.38moles \end{gathered}[/tex]In this case, the addition of 0.81 moles of NaOH neutralizes all the acid in the solution and 0.38 moles of the base will remain unreacted.
So, the concentration of OH- will be 0.38M, and it is necessary to calculate the pOH of the solution, because when OH- is left over, the solution will be basic:
[tex]\begin{gathered} pOH=-log\lbrack OH^-\rbrack \\ pOH=-log(0.38) \\ pOH=0.42 \end{gathered}[/tex]The pOH of the solution is 0.42.
4th) Now with the pOH we can calculate the pH of the solution, with the following formula:
[tex]\begin{gathered} pH+pOH=14 \\ pH+0.42=14 \\ pH=14-0.42 \\ pH=13.58 \end{gathered}[/tex]The pH of the solution is 13.58.
5th) Finally, with the value of pH and the pH formula, we can calculate [H3O+]:
[tex]\begin{gathered} pH=-log\lbrack H3O^+\rbrack \\ 13.58=-log\lbrack H3O^+\rbrack \\ -13.58=log\lbrack H3O^+\rbrack \\ 10^{(-13.58)}=\lbrack H3O^+\rbrack \\ 2.63*10^{-14}M=\lbrack H3O^+\rbrack \end{gathered}[/tex]So, the concentration of H3O+ is 2.63x10^-14M.