Answers
Explanation:
Already it can be noted that both the first and second ionization energy of Ca is greater than that of K. In order to fully understand why this is, we must first look at both elements and their electron configuration.
Ionization energy is basically the energy required to remove the outermost electron in an atom
There are 3 factors that affect the ionization energy:
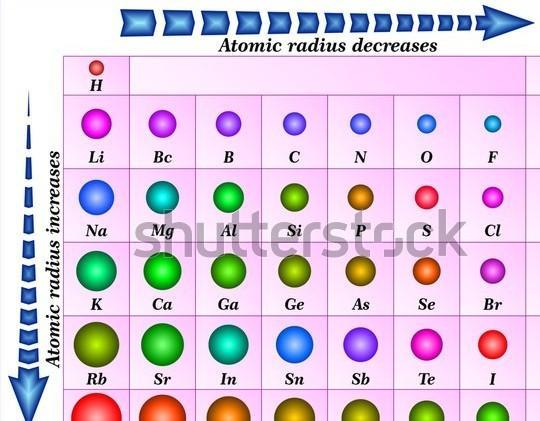
the size of positive nuclear chargedistance of the outermost electron from the nucleusscreening/shielding effect from inner shellsFrom the attachment of the atomic radii trend in the periodic table, we know that atomic radius decreases moving across a period i.e meaning that the outermost shell is closer to the nucleus. And we know that atomic radius increases down a group.
And in the case of K and Ca, we note that Ca has a smaller atomic radii than K, this is because an extra proton and electron is added to the atom that differs it from the previous member, K. Moving from K to Ca, we see that the electron was added to the same shell and there is another proton in the nucleus therefore the attraction of the outermost shell to the nucleus as with the size of the positive nuclear charge increased whilst the shielding effect remained the same for K and Ca, this makes it more difficult now to remove the outer most electron of Ca hence the increased ionization energy as compared to K.
Related Questions
Is there any difference(s) in observation and molarity of the strong base when phenolphthalein or methyl orange is used? If Yes / No account for this reason.
Answers
Yes, the molarity in both the cases will be different if the indicator is changed from methyl orange to phenolphthalein or from phenolphthalein to methyl orange.
Since molarity is defined as the Molar concentration, also known as molarity, amount concentration, or substance concentration, is a unit used to describe the amount of a substance in a solution expressed as a percentage of the volume of the solution or it is the number of moles of solute divided by volume of solution in liters.
So on changing indicator, the number of moles changes as the molar mass is changing, so the molarity and other observations will get changed too and may increase or decrease.
To know more about molarity , please refer:
https://brainly.com/question/14469428
#SPJ9
Determine the molar mass of an unknown monoprotic acid to two decimal places if 16.98 mL of a 0.086 M NaOH solution were used to titrate 0.236 g of the unknown acid
Answers
1 ) Chemical equation
[tex]\text{NaOH + HX}\rightarrow H_2O+Na^++X^-[/tex]HX represents the unknown acid.
2) Moles of NaOH in the reaction
[tex]M=\frac{\text{moles of solute}}{\text{liters of solution}}[/tex]Convert mL into L
[tex]L=16.98mL\cdot\frac{1L}{1000mL}=0.01698L[/tex]Plug in known values in the equation and solve for moles.
[tex]0.086M=\frac{\text{moles of NaOH}}{0.01698L}[/tex][tex]\text{mol NaOH= 0.086M}\cdot0.01698L=0.00146028\text{ mol NaOH}[/tex]3) Moles of the unknown acid that reacted with 0.00146028 mol NaOH
Molar ratio
1 mol NaOH: 1 mol HX
[tex]\text{mol HX=0.00146028 mol NaOH}\cdot\frac{1\text{ mol HX}}{1\text{ mol NaOH}}=0.00146028\text{ mol HX}[/tex]4) Molar mass of the unknown monoprotic acid
[tex]\text{Molar Mass=}\frac{\text{mass of solute (g)}}{moles\text{ of solute}}[/tex]Plug in known values and solve
[tex]\text{Molar Mass}=\frac{0.236\text{ g HX}}{0.00146028\text{ mol HX}}=161.61\text{ g/mol}[/tex]The molar mass of the unknown monoprotic acid is 161.61 g/mol
.
which best corresponds to a strong base?I need to know
Answers
Assuming that the question is considering quantity of OH- ions as strength of base, the most basic compound will be the last one, as it has more OH- being released into the reaction, but the definition of strong base is a base that totally dissociates, but for the purpose of the question, letter D corresponds to a strong base.
I really need help on this problem for chemistry, please help!
Answers
answer and explanation
we are given the balanced reaction and so we begin by calculating the number of mols of each reactant
for Al
mols = mass/Molar mass
= 22.0g / 26.98g/mol
= 0.82 mols
for oxygen
mols = mass / Molar mass
= 26.0g / 16.00g/mol
= 1.6 mols
theerefore
a. the limiting reagent is Al
b. theoretical yield will be determine using the limiting reagent.
from the balanced reaction we see that the mol ratio between aluminum and aluminum oxide is 4:2
therefore
2/4 x0.82 = 0.41 mols of aluminum oxide will form
the mass will be
mass = n x M
= 0.41 mols x 101.96g/mol
= 41.8 grams
c. percentage yield is:
actual yield/ theorectical yield x 100
= 40.0/41.8 x 100%
= 95.7 %
I need to know the answer to this science problem . Please and thank you
Answers
Step 1 - Understanding the types of heat transfer
There are three types of heat transfer: conduction, convection and irradiation. Let's see how each one works:
a) Conduction: is when a very hot substance enters in contact with a cooler substance. The atoms in the hotter substance are moving with a greater velocity and will therefore collide with the atoms of the cooler substance.
b) Convection: it happens especially in liquids and air (fluids). The liquid is warmed up at the bottom first, via conduction. The heated bottom thus changes its density: it becomes less dense, and go up. This process repeats itself several times, warming the whole liquid.
c) Irradiation: when something is put near a heat source, but do not touch it directly. In this case, there's no direct atom collision, but indirect: the rapid atoms of the heat source collide with atoms in the air which then collide with the atoms in the object that is being heated.
Step 2 - Discovering the type of heat transfer in each scenario
In the first scenario, the water at the bottom is in direct contact with the pan. Therefore, water molecules will directly collide with "pan" molecules (probably aluminum atoms or other materials). But there's also convection. The water is not heated only by conduction.
In the second scenario, the hands are near the Bunsen burner, but not directly touching it. What is happening here is then heat irradiation, not conduction.
In the third scenario, the hand is touching the object (the handle of the pan). Even though handles are made of material that poorly conduct heat, it will increase its temperature, at least a little bit. When we touch it, we can feel it is hotter than before. In this case, only conduction is involved (from the handle to our hand). In this case atoms are directly colliding and this is the only source of heat.
Finally, in the last scenario, we also have a case of irradiation: the pan with water is near the heat source, but not directly touching it.
Step 3 - How to set an experiment
In science in general, anytime we want to investigate some effect it is good manners to investigate a system controling all other effects. We want to investigate a variable at a time.
Therefore, while both scenario 1 and 3 involve conduction, scenario 1 also involves convection, which could be a problem to a experiment intending to study conduction only.
The best experiment would be then scenario 3, hands touchind the handle of pan siting in a Bunsen burner.
Answer the following question:Reminder R= .0821. If you have a gas at 6 Atm and 4 L, how many moles are present if the temp is 300 K?
Answers
Answer
0.975609756 mol
Explanation
Given:
Pressure, P = 6 atm
Volume, V = 4 L
Temperature, T = 300 K
Molar gas constant, R = 0.082
What to find:
The moles, n present.
Step-by-step solution:
The mole, n present can be calculated using the ideal gas equation, PV = nRT
6 x 4 = n x 0.082 x 300
24 = 24.6n
Diivide both sides by 24.6
24/24.6 = 24.6n/24.6
n = 0.975609756 mol
Hence, the moles that are present = 0.975609756 mol
A sample of an unknown gas with a molar mass of 85.74 is placed in a vessel with avolume of 1,681 mL at a temperature of 58.6 °C. If the pressure is 5.4 atm, howmany grams of this gas are present?
Answers
Answer
28.58 grams
Explanation
Given:
Molar mass of the gas, M = 85.74 g/mol
Volume, V = 1,681 mL = 1.681 L
Temperature, T = 58.6 °C = (58.6 + 273.15 K) = 331.75 K
Pressure, P = 5.4 atm
What to find:
The mass of the gas in grams present.
Step-by-step solution:
The mass in grams of the gas present can be calculated using the ideal gas equation:
[tex]\begin{gathered} PV=nRT \\ \\ n=moles=\frac{Mass}{Molar\text{ }mass} \\ \\ \Rightarrow PV=\frac{Mass}{Molar\text{ }mass}RT \end{gathered}[/tex]Putting the values of the given parameters and R = 0.0821 atm•L/mol•K into the formula:
[tex]\begin{gathered} 5.4atm\times1.681L=\frac{Mass}{85.74g\text{/}mol}\times0.0821atm•L/mol•K\times331.75K \\ \\ 9.0774atm•L=Mass(0.317665908atm•L/g) \\ \\ Divide\text{ }both\text{ }sides\text{ }by\text{ }0.317665908atm•L/g \\ \\ \frac{9.0774atm•L}{0.317665908atm•L/g}=\frac{Mass(0.317665908atm•L)}{0.317665908atm•L\text{/}g} \\ \\ \Rightarrow Mass=28.58\text{ }grams \end{gathered}[/tex]The mass of the gas in grams present = 28.58 grams.
If there are 1.1 moles of SeCl6 how many moles of Cl2 will be produced? Use up to one decimal point
Answers
ANSWER
There are 3.3 moles of Cl2 in 1.1 moles of SeCl6
EXPLANATION
Given that
The number of moles of SeCl6 is 1.1 moles
Firstly, write a balanced equation of the reaction
[tex]\text{ SeCl}_6\text{ }\rightarrow\text{ Se + 3Cl}_2[/tex]In the reaction above, 1 mole SeCl6 gives 3 moles Cl2
Let x be the number of moles of Cl2
[tex]\begin{gathered} \text{ 1 mole SeCl}_6\text{ }\rightarrow\text{ 3 moles Cl}_2 \\ \text{ 1.1 mole SeCl}_6\text{ }\rightarrow\text{ x moles Cl}_2 \\ \text{ cross multiply} \\ \text{ 1 mole SeCl}_6\times\text{ x moles Cl}_2\text{ = 1.1 mole SeCl}_6\text{ }\times\text{ 3 moles Cl}_2 \\ \text{ Isolate x } \\ \text{ x = }\frac{1.1moles\cancel{SeCl_6}\times\text{ 3 moles Cl}_2}{1mole\cancel{SeCl_6}} \\ \\ \text{ x = 1.1 }\times\text{ 3} \\ \text{ x = 3.3 moles} \end{gathered}[/tex]Therefore, there are 3.3 moles of Cl2 in 1.1 moles of SeCl6
This is a 6 mark question. Please spend some time explaining it, answering it and helping me.
Answers
Answer:
Explanation:
Here, we want to explain the factors responsible for the differences in the polymers presented
We proceed as follows:
a) High melting Point
Although the two polymers contain carbon and hydrogen atoms only, the presence of hydrogen bonding in Polymer Y may lead to it having a high melting point compared to Z.
Other factors that can cause this include the presence of double bonds, aromatic groups or the availability of bulky side groups or branches in the polymer molecule Y.
These and more are responsible for the differences
b) Rigidity and Flexibility
This is due to the existence of some degree of rotation around the atoms' valency. Polymer Z would be flexible due to this.
The absence of this in Polymer Y will make this a difficulty
c) Stretching
How entangled the chain of the polymers are could be responsible for the stretching of the polymers. Since polymer Z is easily stretched, there would be a low degree of entanglement in its chain compared to polymer Y.
Which compound BH3 or BO3 would have polar covalent bonds? How do you know?These are the options in photo
Answers
To solve this problem, let's find the electronegativity difference that we have between each pair of elements. If we look for the electronegativity of B, H and O we might find these values:
B: 1.5 O: 3.4 H: 2.1
So the two bonds that are present in our molecules have a difference of:
O ---- B = 3.4 - 1.5 = 1.9
H ----- B = 2.1 - 1.5 = 0.6
We have two types of covalent bonds, polar and non-polar. When the electronegativity difference is between 1.5 and 0.4 the bond is covalent polar, when it is less that 0.4 is non-polar and when it is higher than 1.5 it is ionic.
In our case, the electronegativity difference between O and B is 1.9, it seems that it would be an ionic bond. But that's not an option. The difference between H and B is 0.6, the polarity of that bond is weak compared to the polarity of the bond between O and B (if we consider that it is covalent).
So the answer to our problem is the first one:
BO₃ would have polar covalent bonds because
Which models of the atom include a structure that is mostly made of empty space?RutherfordThomsonBohrQuantum mechanical
Answers
Rutherford's models
Explanations:What is the electron cloud model?There are known as the region where electrons are found especially in the nucleus.
According to the five basic atomic models which have contributed to the structure of the atom itself, the Rutherford's models of the atom include a structure that is mostly made of empty space compared to thomson that proposed the plum pudding model of the atom
4.A gas occupies 8.7L at a temperature of 29.0°c. What is thevolume at 133°C? (Charles Law)
Answers
11.7L
Explanations
According to Charles law, the volume of a given mass of gas is directly proportional to its temperature provided that the pressure is constant. Mathematically;
[tex]\begin{gathered} v\alpha T \\ v=kT \\ k=\frac{v_1}{T_1}=\frac{v_2}{T_2} \end{gathered}[/tex]where:
• v1 and v2 are the ,initial and final ,volume
,• T1 and T2 are the, initial and final, temperature
Given the following parameters
v1 = 8.7L
T1 = 29.0°C = 29 + 273
T1 = 302K
T2 = 133+ 273 = 406K
Substitute the given parameters into the formula
[tex]\begin{gathered} v_2=\frac{v_1T_2}{T_1} \\ v_2=\frac{8.7L\times406}{302} \\ v_2=\frac{3532.2}{302} \\ v_2=11.7L \end{gathered}[/tex]Hence the volume of the gas at 133°C is 11.7L
Adding up all of the _______ of all of the individual atoms within one molecule of a compound will determine the molecular mass of the compound.A) atomic massesB) electronsC) atomic numbersD) protons
Answers
1) Subatomic particles. There are three main subatomic particles. These subatomic particles are the protons, the neutrons, and the electrons.
The protons and the neutrons are a lot heavier than the electrons. So, they represent most of the weight of an atom.
The protons and the neutrons added together are what we call the mass number.
The correct answer is option A, atomic masses.
When reacting 5.00 g of MgCl2 with 15.0 g of AgNO3 , what is the limiting reagent based on the following equation?MgCl2 (aq) + 2 AgNO3(aq) --> 2 AgCl (s) + Mg(NO3)2 (aq)
Answers
Answer:
[tex]AgNO_3\text{ is the limiting reagent}[/tex]Explanation:
Here, we want to get the limiting reactant
The limiting reactant is the reactant that would produce less amount of the solid precipitate
Firstly, we need to get the number of moles of each of the reactants
To get this, we divide their masses by the molar masses
The molar mass of magnesium chloride is 95 g/mol
The number of moles would be:
[tex]\frac{5}{95}\text{ = 0.053 mol}[/tex]Now, from the equation of reaction, 1 mole of MgCl2 produced 2 moles of AgCl
Then: 0.053 mole of MgCl2 will produce 2 * 0.053 mol = 0.106 mol AgCl
For AgNO3, the molar mass is 170 g/mol
The number of moles would be:
[tex]\frac{15}{170}\text{ = 0.088 mol}[/tex]Now, looking at the equation of reaction:
2 moles of AgNO3 produce 2 moles of AgCl
0.088 mol AgNO3 will also produce 0.088 mol AgNO3
Now, looking at the values of the number of moles of AgCl produced, we can see that AgNO3 produces less of the product
This means that AgNO3 is the limiting reactant
A piece of sodium metal reacts completely with water. The hydrogen gas generated is collected over water at 25oC. The volume of the gas is 252mL measured at 1.00atm. Calculate the number of grams of hydrogen that was collected
Answers
The mass or number of grams of hydrogen that was collected in the experiment is 0.02 g.
What is the number of moles of hydrogen gas collected at the given conditions of temperature and pressure?The number of moles of hydrogen gas collected at the given conditions of temperature and pressure is calculated using the ideal gas equation as follows:
PV = nRT
Where;
P is pressure = 1.00 atm
V is volume = 252 mL or 0.252 L
n is the number of moles = ?
R is molar gas constant = 0.082 L.atm/K.mol
T is temperature = 273 + 25 K
T = 298 K
n = PV/RT
n = 1 * 0.252 / (298 * 0.082)
n = 0.01 moles
1 mole of hydrogen gas has a mass of 2.0 g
mass of 0.01 moles of hydrogen = 2 * 0.01
Mass of 0.01 moles of hydrogen = 0.02 g
Learn more about ideal gas equation at: https://brainly.com/question/27870704
#SPJ1
Using the Gay-Lussac LawIf I have 7.7 moles of gas at a pressure of 0.09 atm at a temperature of 56 C°, what is the volume of the container that the gas is in?
Answers
ANSWER
EXPLANATION
Given that;
The number of moles of the gas is 7.7 moles
The pressure of the gas is 0.09 atm
The temperature of the gas is 56 degrees Celcius
Follow the steps below to find the volume of the gas in the container
Step 1; Convert the temperature to degrees Kelvin
[tex]\text{ T K = t}\degree C\text{ + 273.15}[/tex][tex]\begin{gathered} \text{ T K = 56 + 273.15} \\ \text{ T K = 329.15K} \end{gathered}[/tex]Step 2; Apply the ideal gas equation
[tex]\text{ PV = nRT}[/tex][tex]\begin{gathered} \text{ Recall, that R = 0.08205 L atm mol}^{-1}K^{-1} \\ \text{ 0.09 }\times\text{ V = 7.7 }\times\text{ 0.08205 }\times\text{ 329.15} \\ \text{ 0.09V = 207.95} \\ \text{ Divide both sides by 0.09} \\ \text{ V = }\frac{\text{ 207.95}}{\text{ 0.09}} \\ \text{ V = 2310.58 Liters} \end{gathered}[/tex]Therefore, the volume of the container is 2310.58 liters
when 14.12 moles of mercury (ll) oxide decomposes, how many moles of oxygen gas will form ?
Answers
ANSWER
The number of moles of oxygen formed is 7.06 moles
EXPLANATION
Given that
The number of moles of mercury (II) oxide is 14.12 moles
Follow the steps below to find the number of moles of oxygen
Step 1; Write the balanced equation for the decomposition of the reaction
[tex]\text{ 2HgO }\rightarrow\text{ 2Hg}_{(s)}\text{ + O}_{2(g)}[/tex]In the reaction above, 2 moles HgO decompose to produce 2 moles Hg and 1 mole O2
Step 2; Find the number of moles of oxygen using a stoichiometry ratio
Let x represents the number of moles of oxygen
[tex]\begin{gathered} \text{ 2 moles HgO }\rightarrow\text{ 1 mole O}_2 \\ \text{ 14.12 moles HgO}\rightarrow\text{ x mole O}_2 \\ \text{ cross multiply} \\ \text{ 2 moles HgO }\times\text{ x mole O}_2\text{ }=\text{ 1 mole O}_2\times\text{ 14.12 moles HgO} \\ \text{ Isolate x} \\ \text{ x = }\frac{1\text{ mole O}_2\times14.12moles\cancel{HgO}}{2moles\cancel{HgO}} \\ \text{ x = }\frac{14.12}{2} \\ \text{ x = 7.06 moles} \end{gathered}[/tex]Therefore, the number of moles of oxygen formed is 7.06 moles
What part of a cell provides instructions for the processes within the cell
DNA _ Chloroplasts _ Cell wall or Glucose
Answers
The information for the cellular process is provided by DNA. DNA has genes on it, so by expressing the gene, the characteristics are expressed in the cell.
What is a gene?
Genes are present in the DNA of both prokaryotes and eukaryotes. The information of the cell is present in the gene, and it is expressed by the processes of transcription and translation in the cell.
Because of differences in gene expression, different cells, such as the nerve cell, the cardiac cell, the epithelial cell contain different proteins. Not all genes are expressed in all cells of the body. Only those genes are expressed that are transcriptionally activated and make mRNA.
Hence, DNA provides the information to the cell for the cellular process.
Learn more about the gene, here
https://brainly.com/question/411085
#SPJ1
what is the percent composition of h and o in H2O2
Answers
Percent compositions:
[tex]\begin{gathered} Hydrogen:\frac{2}{34}\times100=5.9\% \\ \\ Oxygen:\frac{32}{34}\times100=94.1\% \end{gathered}[/tex]PLEASE HELP
Balance the chemical reaction
using an atom inventory.
What is the coefficient for
oxygen?
4NH3 + ? O2 ---> ?NO + ? H2O
Answers
The balance chemical equation will be 4NH3 + 5 O2 → 4NO + 6H2O and the coefficient for water will be 5.
The given unbalanced chemical equation is :
4NH3 + O2 → NO + H2O
It can be seen that number of nitrogen atom in reactant side is 4. By multiplying by 4 as the coefficient of NO , multiplying by 5 as the coefficient of oxygen and multiplying by 6 as the coefficient of H2O. The equation will be balanced.
The balanced chemical equation will be :
4NH3 + 5O2 →4 NO + 6H2O
Therefore, the the coefficient for water will be 5.
To know more about balance chemical equation
https://brainly.com/question/7402009
#SPJ1
What is the pH of a solution where [OH-] = 5.6 × 10-4 M?
Answers
The pH value indicates the concentration of H+ ions in the solution. We are given the value of the concentrations of the OH- ions, so we can find the pOH using the following equation:
[tex]pOH=-log\lbrack OH^-\rbrack[/tex]The sum of pH and pOH will always be equal to 14, so we find the pH by clearing it from the following relationship:
[tex]pH+pOH=14[/tex]So, pOH value will be:
[tex]pOH=-log\lbrack5.6\times10^{-4}\rbrack=3.3[/tex]The pH will be:
[tex]\begin{gathered} pH=14-pOH \\ pH=14-3.3=10.7 \end{gathered}[/tex]The pH of the solution will be 10.7
Which of the following is not an empirical formula?H2O2HOH2OH3O
Answers
An empirical formula is the simplest form of a formula, that is, the subscripts are simplified.
Therefore, the answer is H2O2 because it's not the simplest form.
Select the correct structure thatcorresponds to the name.2-hexyneA.CH3CH₂CH₂C=CCH3B. CH3C CCH₂CH₂CH3C. both
Answers
Answer
c. both
explanation
both structures have a triple bond on the second carbon and the have 6 carbons in the carbon chain
Determine the molarity of a solution with a volume of 707. mL and 0.610 mol of solute dissolved.Answer: ____ M
Answers
Molarity or molar concentration is a measure of the concentration of a chemical species, of a solute in a solution, using as units, number of moles, and volume in liters. The formula for Molarity is:
M = n/V
Where:
n = number of moles, 0.610 moles
V = volume in Liters, 0.707
Now we add these values into the formula:
M = 0.610/0.707
M = 0.863 M is the molarity
Calcium reacts with Aluminum chloride to form Calcium chloride and Aluminum. If you react 100 grams of Aluminum chloride with Calcium, how many grams of Aluminum are produced?
Answers
38.95 g of Al is produced on reacting 100 g of Aluminium chloride with Calcium.
We need to write a balanced equation for the reaction. This is illustrated below:
2AlCl₃ + 3Ca —> 3CaCl₂ + 2Al
Molar Mass of AlCl₃
= 27 + (3x35.5)
= 27 + 106.5 = 133.5g/ mol
Mass of AlCl₃ from the balanced equation
= 2 x 133.5g
= 267g
Molar mass of Al= 26
Mass of Al from balanced equation= 2×26
= 52
Molar Mass of CaCl₂ = 40 + (2 x 35.5)
= 40 + 71 = 111g/mol
Mass of CaCl₂ from the balanced equation
= 3 x 111 = 333g
From the equation,
133.5g of AlCl₃ produced 52g of Al
Therefore, 100g of AlCl₃ will produce
= (100 x 52)/133.5
= 38.95g of Al
So, 38.95 g of Al is produced on reacting 100 g of Aluminium chloride with calcium.
To know more about stochiometric reactions, please refer:
https://brainly.com/question/29042675
#SPJ1
6. A sample of nitrogen gas weighs 130 g. Write the chemical formula for nitrogen gas. Howmany molecules of elemental nitrogen is this? How many atoms of nitrogen are in this sample?
Answers
Chemical formula for nitrogen gas is N₂.
To find the number of molecules in the given sample, we have to convert the mass of the sample to moles by using the molecular mass of elemental nitrogen (N₂).
[tex]130gN_2\cdot\frac{1molN_2}{28gN_2}=4.64molN_2[/tex]Now, we have to use Avogadro's number (6.022x10^23) that indicates the number of molecules in one mole of substance:
[tex]4.64molN_2\cdot\frac{6.022\times10^{23}molecules}{1molN_2}=2.79\times10^{24}molecules[/tex]It means that there are 2.79x10^24 molecules of elemental nitrogen.
To find the number of atoms we just have to multiply the number of molecules by 2, which is the number of atoms of nitrogen per molecule of elemental nitrogen:
[tex]2.79\times10^{24}molecules\cdot\frac{2atoms}{1molecule}=5.59\times10^{24}atoms[/tex]There are 5.59x10^24 atoms of nitrogen in the sample.
Select the statements that are true about electronegativity between elements, and their type of bond.A.If the difference is over 1.0, an ionic bond will form.B.If the difference in electronegativity is 0 to 0.5, the bond will be polar covalent.C.If the difference in electronegativity is from 0.6 to 1.7, the bond will be polar and covalent.
Answers
Answer:
C. If the difference in electronegativity is from 0.6 to 1.7, the bond will be polar and covalent.
Explanation:
Let's see the type of bondings based on the electronegativity differences between atoms:
[tex]\begin{gathered} less\text{ than 0.4 : Nonpolar covalent.} \\ 0.4\text{ - 1.7 :}Pola\text{r covalent.} \\ Greater\text{ than 1.7: Ionic.} \end{gathered}[/tex]So based on this, the answer would be C. If the difference in electronegativity is from 0.6 to 1.7, the bond will be polar and covalent.
The Croatian seismologist, Andrija Mohorovicic, discovered a boundary change in the Earth's layers. Please explain how he discovered this using two to three sentences using your best grammar.
Answers
The Croatian seismologist, Andrija Mohorovicic, discovered a boundary change in the Earth's layers because he noticed that P-waves were refracted at the boundary.
Who is a seismologist?Seismologists are described as Earth scientists, specialized in geophysics, who study the genesis and the propagation of seismic waves in geological materials.
Andrija Mohorovicic realized that as one crosses the boundary the predominant mineral composition of the rock changes, and the minerals on the mantle side enable seismic waves to travel faster.
Learn more about Seismologists at: https://brainly.com/question/676287
#SPJ1
How many moles are in 91.5 grams of Helium?
Answers
In order to find the number of moles with a given mass of Helium, we need to use its molar mass, which is 4.0026g/mol, therefore we will have:
4.0026g = 1 mol of Helium
91.5g = x moles of Helium
x = 22.86 moles of Helium in 91.5 grams
A compound of molecular mass 28 has a molecular formula CxHy. What is the value of x and y if its empirical formula is CH?
Answers
Answer
x = 2, y = 2
Explanation
The empirical formula is the simplest whole number ration that defines constituent atoms in a species.
The molecular formula is always a multiple of the empirical formula.
Since the empirical formula of the compound i given to be CH, ithen its molecula formula is:
[tex]Molecular\text{ }formula=n\times(empirical\text{ }formula)[/tex]From the periodic table, the molar masses of (H = 1.00784, C = 12.011)
Thus, the molecular formula is
[tex]\begin{gathered} Molecular\text{ }formula=28\text{ }g\text{/}mol=n(12.011+1.00784)\text{ }g\text{/}mol \\ \\ n=\frac{28}{12.011+1.00784}=\frac{28}{13.01884}=2.150729251\approx2 \end{gathered}[/tex](CH)n = (CH)₂ = C₂H₂
Therefore, CxHy = C₂H₂. The value of x and y is 2 and 2 respectively.
The value of x and y if its empirical formula is CH is x = 2, y = 2