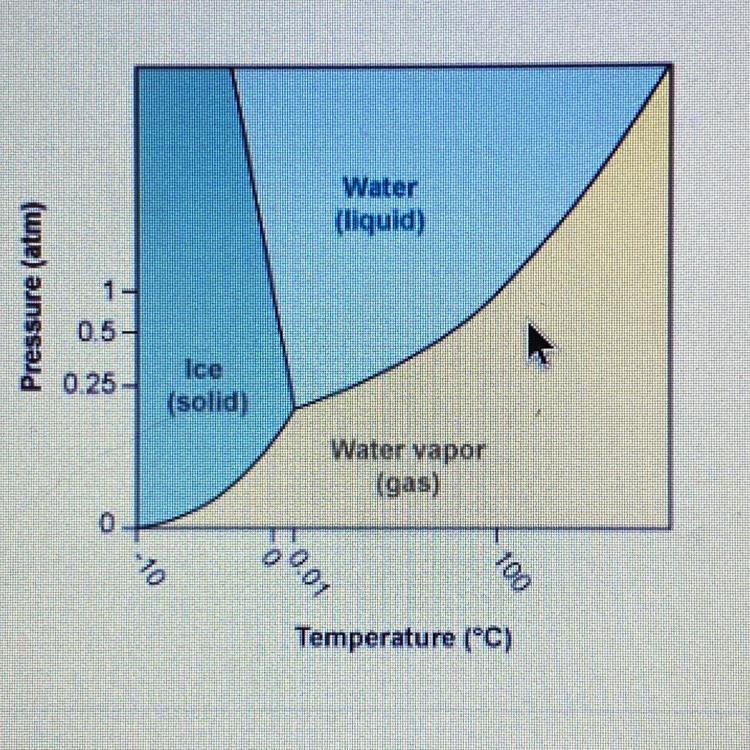
According to the phase diagram for H₂O, what happens to the phases ofwater at 0.5 atm pressure as the temperature is decreased from 110°C to -10°C?A) water changes from a gas to a solid to a liquidB) Water changes from a solid to a liquid to a gasC) water changes from a liquid to a gas to a solidD) water changes from a gas to a liquid to a solid
Answers
Answer:
[tex]D[/tex]Explanation:
Here, we want to get what happens to the phases of water as the temperature is decreased at 5 atm
From what we have in the question, we would be moving from left to right
What would happen here is that we move from the gaseous state to the liquid and then to the ice state
This means we have a change from a gas to a liquid then a solid
Related Questions
Consider the equation 2 C2H6 + 7 O2 ---> 4 CO2 + 6H2O
How many grams of H2O are produced from 268.5 grams of C2H6 ?
Answers
The equation 2 C₂H₆ + 7 O2 ⇒ 4 CO₂ + 6H₂O. 482.6 gram of H₂O are produced from 268.5 grams of C₂H₆.
What is ethane ?A saturated hydrocarbon that exists in a gaseous form is ethane. Methane is the simplest alkane, with ethane coming in second. It has 6 hydrogen atoms and 2 carbon atoms. Ethane's chemical formula is CH
With the chemical formula C₂H₆, ethane is an organic chemical substance. Ethane is a colorless and odorless gas at ordinary temperature and pressure.
Ethane is separated from natural gas on an industrial scale, and it is produced as a by-product of the petrochemical process used to refine crude oil.
268.5 gram of C₂H₆ × ( 1 mole C₂H₆ ÷ 30.07 gram of C₂H₆ ) × ( 6 mole of H₂O ÷ 2 mole C₂H₆ ) × ( 18.015 gram of H₂O ÷ 1 mole of H₂O )
= 482.6 gram of H₂O.
Thus, the equation 2 C₂H₆ + 7 O2 ⇒ 4 CO₂ + 6H₂O. 482.6 gram of H₂O are produced from 268.5 grams of C₂H₆.
To learn more about ethane follow the link below;
https://brainly.com/question/16156775
#SPJ1
Determine the mixture in equimolar quantities, if the PH is equal to, less than or greater than 7.A) A weak base and a strong acidB) a strong Base and a strong acidC) A strong base and an acid
Answers
We need to determine how is the PH in the given mixtures:
A) A weak base and a strong acid:
Acid and base react to form an acidic solution and, being acidic, this results in a solution with a pH lower than 7
B) A strong base and a strong acid:
When mixing a strong base and a strong acid, neutralization occurs, that is, a neutral pH or equal to 7 is obtained
C) A strong base and acid:
If we have a weak acid, when adding a strong base the pH approaches neutrality without sudden changes, but once the acid has been neutralized, just add a few drops of excess soda to obtain a sudden increase in pH as if there were only base. free. In this case, at the equivalence point the pH> 7
ANSWER:
A) Less than 7
B) Equal to 7
C) Greater than 7
How to balance a chemical equationThe question is blank Al + cl2 = Alcl3
Answers
First, we write our reaction:
Al + Cl2 ==> AlCl3
To balance a reaction similar to this one, we must focus on having the same number of atoms on each side, I mean on the left side and on the right side of the reaction.
As we can see, we have 2 Cl on the left and 3 on the right, therefore we try to have the same number of this atom, multiplying by a pair number and an odd number respectively e.g. by 3 and 2.
Al + 3 Cl2 ==> 2 AlCl3
After that, we have 6 Cl on both sides. Then, we must go on with Al, and the only thing we should do is multiply on the left by 2 because we already have 2 Al on the right side.
2 Al + 3 Cl2 ==> 2 AlCl3
With this, we will have 2 Al and 6 Cl in total
Answer: 2 Al + 3 Cl2 ==> 2 AlCl3
35 ml of liquid has the mass of 41.6 g what is the density
Answers
INFORMATION:
We know that:
- 35 ml of liquid has the mass of 41.6 g
And we must find its density
STEP BY STEP EXPLANATION:
To find it, we must use the following formula
[tex]density=\frac{mass}{volume}[/tex]From given information:
- mass of the liquid: 41.6 g
- volume of the liquid: 35 ml
Now, replacing the values in the formula
[tex]\begin{gathered} density=\frac{41.6g}{35ml} \\ \text{ Simplifying,} \\ density=1.19\frac{g}{ml} \end{gathered}[/tex]Finally, the density of the liquid is 1.19 g/ml
ANSWER:
The density of the liquid is 1.19 g/ml
an element with the valence electron configuration 3s2 3p2 belongs to group
Answers
Answer:
Group 4A (Group IVA)
The answer is B
Explanation:
An element with valence electron configuration:
[tex]3s^23p^2[/tex]has 4 valence electrons.
Since the valence shell is n = 3 it is in Period 3. Since there are 4 valence electrons (2 in 3s and 2 in 3p) it is in Group 4A
Which of the following is an example of engineering, not science?
A. Designing a radiation containment system
B. Observing the effect of radiation on photographic film
C. Determining the effects of radiation on plants
D. Studying different types of radiation
Answers
Answer:
A. Designing a radiation containment system
Explanation:
Engineers invent, create, design, and maintain machines, building/structures, and software data.
During her presentation, Zahara was asked several questions from the audience and to provide the molecular formula for molecules that the audience had questions about. Help Zahara classify whether the following items are an element, a compound, a homogenous mixture, or a heterogenous mixture: Iron
Answers
Iron is an element.
This is because Iron consists of a single type of atom.
QUESTION 15The reaction below is an example of which type of reaction?1 Na20 (aq) + 1 H20 (1) 2 NaOH (aq)()→O Double DisplacementO Synthesis (or Combination)O Single DisplacementO CombustionO Decomposition
Answers
Answer
Synthesis
Explanation
Synthesis reaction is when reactants combine to
The state of matter that has an indefiniteshape, indefinite volume, and highcompressibility isA. solidB. liquidC. gas
Answers
Answer:
Explanation:
Here, we want to answer a question of state of matter
While solids have a definite shape and are incompressible under normal conditions, liq
Imagine two different substances that are both pure, meaning they each contain only one
type of matter. Each substance has a different temperature.
Which two properties determine which substance has the higher temperature?
the mass of one particle in each substance
the average speed of the particles in each substance
the speed at which each substance is moving
the kinetic energy of the slowest particle in each substance
Answers
two different substances that are both pure, meaning they each contain only one type of matter. two properties determine which substance has the higher temperature is the kinetic energy of the slowest particle in each substance
What are the types of energy ?The energy can be defined as ability to work or produce action and / or movement and manifests itself in many different ways, such as body movement, heat, electricity, etc.
The different types of energy include Kinetic energy which is associated with the movement of bodies. Potential energy which is stored by virtue of a body's position relative to its surface is also called gravitational potential energy.
Thermal Energy or Heat can be defined as the energy associated with the kinetic energy of the molecules that make up an element, it can be manifested if there is a temperature difference between two bodies.
For more details regarding energy, visit
brainly.com/question/28869293
#SPJ1
What is the molecular formula of a compound given the molar mass of the compound is 34.04 gram and the empirical formula isNH?(A)NHB) N4H4N2H6N2H2
Answers
1) List the known and unknown quantities.
Molar mass: 34.04 g.
Empirical formula: NH.
Molecular formula: unknown.
2) Find the molar mass of the empirical formula.
N: 14.007 g/mol.
H: 1.008 g/mol.
N + H = 14.007 + 1.008 = 15.015 g/mol.
3) Divide the molar mass by the molar mass of the empirical formula
[tex]X=\frac{34.04}{15.015}[/tex][tex]X=2.27[/tex]Multiply the subscripts of the empirical formula by 2.
[tex]N_2H_2[/tex].
How does Boron achieve a noble gas electron configuration?
Answers
Boron gas can achieve noble gas configuration by losing or gaining electrons.
Noble gases were substances that have completed respective octets and have entirely filled respective valence shells. Helium (He), Neon(Ne), Argon(Ar), Krypton(Kr), Xenon (Xe), as well as Radon, are still examples of noble gases.
Atoms can adopt a noble gas configuration whether by acquiring new electrons or by giving and receiving electrons already present in even their own outermost shell.
The atomic number of Boron is 5 . the electronic configuration of B is 2,3. When it will gain 5 electrons with another atom or lose 3 electrons then it will attain a noble gas configuration.
To know more noble gas configuration
https://brainly.com/question/27848059
#SPJ1
4.A 250 mL sample of HCl solution reacts with 2.5 g of Al according to the following reaction. Determine the concentrationof the HCI. Show your work for credit.6HCl(aq) + 2Al(s) -----> 2AICI, (aq) + 3H:(9)
Answers
In this question, we have the following reaction:
6 HCl + 2 Al -> 2 AlCl3 + 3 H2
We have:
250 mL of HCl
2.5 g of Al
And we want to find the molar concentration of HCl
One way of doing it is by finding the number of moles of HCl present in this solution, we can do that by using the number of moles of Al and after that, comparing the molar ratio of HCl and Al. To find the number of moles of Al, we will use its molar mass 27g/mol
27g = 1 mol
2.5g = x moles
x = 0.0926 moles of Al in 2.5 grams
Now by looking at the properly balanced reaction, we can see that the molar ratio between HCl and Al is 6:2, so if we have 0.0926 moles of Al, we will have:
6 HCl = 2 Al
x HCl = 0.0926 Al
x = 0.2778 moles of HCl
Now that we have the volume and the number of moles, we can find the molar concentration of HCl, using the Molarity formula:
M = number of moles/volume in liters
M = 0.2778/0.250
M = 1.11 is the molar concentration of HCl in this given solution
What is the wavelength of the wave shown below?A.0.5 cmB.2.4 cmC.0.4 cmD.1.0 cm
Answers
The definition of wavelength is the distance between two identical points, like the crests of the waves, in a cycle of a waveform, so the distance between two crests, which is the highest point of the wave, this is wavelength
In our question, we have that one crest starts at 1.5 cm and the other starts at 2.5 cm, therefore the wavelength will be 1.0 cm of distance, letter D
What mass of H atoms is contained in 50.7 g
of NH3?
Answer in units of g.
Answers
The mass of hydrogen present in 50.7 grams of ammonia is equal to 8.95 g.
What is a mole?A mole is a scientific unit that is used to determine the huge number of quantities of atoms, molecules, ions, etc. The mass of the 1 mole of any element is called atomic mass and that of one mole of any compound is called molar mass.
The number of entities present in 1 mole was found to be equal to 6.023 × 10 ²³ per mole which is known as Avogadro’s constant.
Given, the mass of ammonia = 50.7 g
The mass of the one mole of ammonia = 17 g/mol
17 g of ammonia contain the mass of hydrogen = 3 g
50.7 g of ammonia contains a mass of hydrogen = (3/17) × 50.7 = 8.95 g
Learn more about the mole, here:
brainly.com/question/26416088
#SPJ1
ellusPerform the followingmathematical operation, andreport the answer to theappropriate number ofsignificant figures.5.1 - 2.3685 =-
Answers
1) Uncertainty in substraction. the result must have the same number of decimal places as the one with less.
5.1 - 2.3685 = 2.7315
The result must have one decimal place. Since the second decimal place (3) is smaller than five we round down.
The result is 2.7.
How many atoms are in Hg3 PO 42
Answers
If 1219 Joules of heat raise the temperature of 250g of metal by 64degreesC. What is the specific heat of the metal in J/g degreesC
Answers
To answer this question, we have to use the following formula:
[tex]Q=Cp\cdot m\cdot\Delta T[/tex]Where Q is the heat, Cp is the specific heat, m is the mass and ΔT is the difference in temperature.
In this case, we know the heat, the mass and the difference in temperature and we need to find the specific heat. Solve the given equation for Cp:
[tex]Cp=\frac{Q}{m\cdot\Delta T}[/tex]Replace for the given values and solve:
[tex]\begin{gathered} Cp=\frac{1219J}{250g\cdot64\degree C} \\ Cp=0.076J/g\degree C \end{gathered}[/tex]It means that the specific heat of the metal is 0.076J/g°C.
Determine the overall and specific shapes around each of the specified atoms in the following molecule. Then, draw a 3D VSEPR structure on the right.
Answers
Explanation
A) Trigonal planar
B) Trigonal pyramid
C) Tetrahedral
D) Linear
E) Linear
F) Tetrahedral
G) Linear
Calculate the mass of iron (0.444 J/goC) that can be heated from 5.4°C to 22.8°C using +41.6 J of energy
Answers
5.38 g is the mass of iron (0.444 J/goC) that can be heated from 5.4°C to 22.8°C using +41.6 J of energy
q=+41.6 J
c=0.444 J/goC
Δt=17.4
q=mcΔt
m=q/cΔt
m=+41.6 J /0.444 J/goC×17.4
m=5.38 g
Mass is one of the fundamental quantities in chemistry and the most fundamental property of matter. The quantity of matter in a body is referred to as its mass. The SI unit of mass is the kilogram.
The mass of a body is constant at all times. only when a body receives or loses a considerable amount of energy, which happens seldom. For instance, a nuclear reaction causes the mass of the material to decrease because a very little amount of matter is converted into a very high amount of energy.
To know more about mass visit : https://brainly.com/question/19694949
#SPJ9
A 120 mg sample of technetium-99m is used for a diagnostic test. If technetium-99m has a half-life of 6.0 h, how many milligrams of the technetium-99m sample remains active 23 h after the test?
Answers
Answer:
Explanation:
Here, we want to get the number of mg of the atom that would remain
Half-life refers to the time taken for exactly half the mass of a radioactive isotope to be lost to radiation
From the question, the half-life is 6 hours
During the first six hours, we have a mass of 60 mg left
In the next 6 hours, which is the second half-life, we have 30 mg left
In the next 6 hours, which is the third half-life, we have 15 mg left
Now, for the next 5 hours, there will not be a complete decay
Thus, we get the decay constant using the following:
[tex]\begin{gathered} t_{\frac{1}{2}}\text{ = }\frac{0.693}{k} \\ \\ 6\text{ = }\frac{0.693}{k} \\ \\ k\text{ = }\frac{0.693}{6}\text{ = 0.1155 h}^{-1} \end{gathered}[/tex]Mathematically:
9) Determine the molecular formula for ibuprofen, a common headache remedy.
Ibuprofen contains a percent composition of 75.7% C, 8.80% H and 15.5% O
and has a molar mass of 206g/mole.
Answers
Ibuprofen has a molecular formula of C13H18O2, meaning it has 13 carbon atoms, 18 hydrogen atoms, and 2 oxygen atoms.
The molar mass of ibuprofen (206 g/mol) is determined by adding up the atomic masses of each element in the molecule:
C (12.01 g/mol) + H (1.01 g/mol) + O (16.00 g/mol) = C13H18O2
Ibuprofen's percent composition (75.7% C, 8.80% H, 15.5% O) can be used to determine the number of each type of atom in the molecule:
13 C atoms x 100% = 75.7% C
8.80 H atoms x 100% = 8.80% H
15.5 O atoms x 100% = 15.5% O
Thus, ibuprofen has 13 carbon atoms, 8 hydrogen atoms, and 15 oxygen atoms.
To know more about antacids, click below:
https://brainly.com/question/13102012
#SPJ1
Match each term to its description.MatchExcess reactantLimiting reactantTheoretical yieldA) Product amount that can be made by the given amount of reactantsB) Reactant that determines how much of the product can be madeC) Reactant that is not all used up in the reaction
Answers
The terms excess reactant and limiting reactant refer to how much of each reactant is available to react. Therefore, by its name we can understand that limiting reactant really limits the reaction, that is, it determines how much product can be made (B).
Excess reactant is also very literal, as it refers to the reactant in excess, that is, the one that is not all used up in the reaction (C).
And Theoretical yield refers to the amount of product that is produced based on the amount of reactants that really react, that is, the amount of product that can be made by the given amount of reactants (A)
In conclusion,
A) Theoretical yield.
B) Limiting reactant.
C) Excess reactant.
For the complete redox reaction given, write the half-reactions & identify the oxidizing & reducing agents.4Fe + 3O 2 -> 2Fe 2 O 3
Answers
4Fe + 3O2 ==> 2Fe2O3
What is the solution's freezing point: 15 g of CH4N2O (Molar mass = 60.055 g/mol) in 200. g of H2O? (Kf = 1.86 (°C·kg)/mol)
Answers
Ok to answer this question we firsst need to fin the number of mol of Urea (CH4N2O). to do this we simply :
1 mol of urea =15/60.055 = 0.25mol
therefore 200g of water contain 0.25mol
the next step is to determine the malality of our solution in 200g of water, to do this we say:
200 g = 1Kg/1000g = 0.2kg
therefor 0.25mol/0.2Kg = 1.25mol/kg
and from the equation:
we know that i = 1
we are given Kf
b is the molality that we just calculated
therefore;
the solutions freezing point is -2.325°C
The freezing point of the given solution was calculated to be -2.325 °C. The freezing point is the point at which a liquid changes from a liquid to a solid under atmospheric pressure.
The two phases, liquid and solid, exist in equilibrium at the freezing point. At the freezing point, both solid and liquid exist at the same time.
Given,
15 grams of CH₄N₂O is dissolved in 200 grams of water.
1 mol of urea = 60 grams
so 15 grams of urea will be found in 15/60 = 0.25 moles
So 0.25 moles of urea is present in 200 grams of water.
To determine the molality of the solution
200gm/1000gm = 0.2 kg water
0.25 moles/0.2 kg = 1.25 kg/mol
So to calculate the freezing point of the solution we use the formula:
ΔT = i× Kf × b
Given Kf = 1.86 (°C·kg)/mol), b is the molality of the solution that is = 1.25 kg/mol, and i = 1
ΔT = 1 × 1.86 × 1.25 = 2.325 °C
ΔT = 0 °C - 2.325 °C = -2.325 °C
To learn more about the freezing point, refer to the link:
https://brainly.com/question/31357864
#SPJ6
How many neutrons are found in the element Al?
Answers
Answer:Neutron = 27 – 13 = 14. Therefore, an aluminum atom has fourteen neutrons. Based on the atomic number, mass number, and neutron number of the element, three things can be considered.
Explanation:
N2 + 2O2 → N2O4If 23.6 grams of N2O4 was produced, how many moles of O2 were required?
Answers
Step 1 - Understanding the stoichiometry of the reaction
The given reaction is:
[tex]N_{2(g)}+2O_{2(g)}\rightleftarrows N_2O_{4(g)}[/tex]Note that 1 mole of Nitrogen gas react with 2 moles of Oxygen gas thus producing 1 mole of N2O4 gas. Since this is a fixed relation, we can state that:
each 2 moles of O2 produce 1 mole of N2O4
Step 2 - Converting the relation in moles to a relation in grams
We can convert the relation in moles between O2 and N2O4 to a relation in grams by multiplying each number of moles by the respective molar masses of the substances (32 g/mol for O2; 92 g/mol for N2O4):
[tex]\begin{gathered} O_2\to2\times32=64\text{ g} \\ N_2O_4\to1\times92=92\text{ g} \end{gathered}[/tex]We can state therefore that:
64 g of O2 (2 moles) produce 92 g of N2O4
This is like a cake recipe. It is a fixed proportion in grams, and we'll be using it to solve the exercise.
Step 3 - Finding the required moles of O2
Now that we have found a "recipe" for the reaction, we can use it to predict how much O2 would be needed. Let's remember that, in step 2, we have discovered that 2 moles of O2 produce 92 g of N2O4.
Therefore, to produce 23.6 grams, we can set the following proportion:
[tex]\begin{gathered} 2\text{ moles of O2 produce ---- 92 g of N2O4} \\ x\text{ ------------------ 23.6 g of N2O4} \\ \\ x=\frac{23.6\times2}{92}=0.51\text{ moles of O2} \end{gathered}[/tex]Therefore, 0.51 moles of O2 would be required.
With all the steps please!For a 0.2 M aqueous solution of sodium hydroxide, calculate the following:a) pHb) pOHc) [H+]d) [OH-]
Answers
Answer:
a) pH= 13.3
b) pOH= 0.7
c) [H+]= 5.01*10^-14M
d) [OH-]= 0.2M
Explanation:
The formula of sodium hydroxide is NaOH. In the molecule there are Na+ ions and OH- that dissociates like this:
[tex]\text{ NaOH}\rightarrow\text{ Na}^++\text{ OH}^-[/tex]That means that 1 mole of NaOH dissociates into 1 mole of Na+ ions and 1 mole of OH-.
So, for a 0.2M solution, 0.2 moles of NaOH will dissociate into 0.2 moles of Na+ ions and 0.2 moles of OH-.
d) [OH-]
From the dissociation of NaOH we know that the concentration of OH- is [OH-]=0.2M.
b) pOH
With the concentration of OH-, we can calulate the pOH:
[tex]\begin{gathered} pOH=-log\lbrack OH^-] \\ pOH=-log(0.2) \\ pOH=0.7 \end{gathered}[/tex]So, the pOH is 0.7.
a) pH
Knowing the pOH and the following formula, we can calculate the pH of the solution:
[tex]\begin{gathered} pH+pOH=14 \\ pH+0.7=14 \\ pH=14-0.7 \\ pH=13.3 \end{gathered}[/tex]The pH of the solution is 13.3.
c) [H+]
Now that we know thw pH of the solution, we can calculate the concentration of H+:
[tex]\begin{gathered} pH=-log\lbrack H^+\rbrack \\ 13.3=-log\lbrack H^+\rbrack \\ 10^{(-13.3)}=\lbrack H^+\rbrack \\ 5.01*10^{-14}=\lbrack H^+\rbrack \end{gathered}[/tex]So, the concentration of H+ is 5.01*10^-14M.
A gas sample has 1.65 moles and occupies 3.50 L. What volume will it occupy with 2.60 moles?
Answers
answer and explanation
Given:
V1 = 3.50 liters
n1 = 1.65 mols
n2 = 2.60 mols
V2 = unknown
solution
to determine the volume that will be occupied by 2.60 mols we can use to following equation
V1/V2 = n1/n2
and we can rearrange the equation to make V2 subject of the formula like so:
V2 = V1n2/n1
= 3.50L x 2.60mols/1.65 mols
= 5.5 liters
2.60 mols will occupy a volume of 5.5 liters
What is Le Chatelier's principle used for? OA. To determine what effect a change will have on a mixture at equilibrium. B. None of these OC. To determine the magnitude of a change in equilibrium. D. All of these
Answers
Le Chatelier's principle is used to determine what effect a change will have on a mixture at equilibrium.
It describes the effect that a mixture suffers when it is exposed to a change.
It means that the correct answer is A.