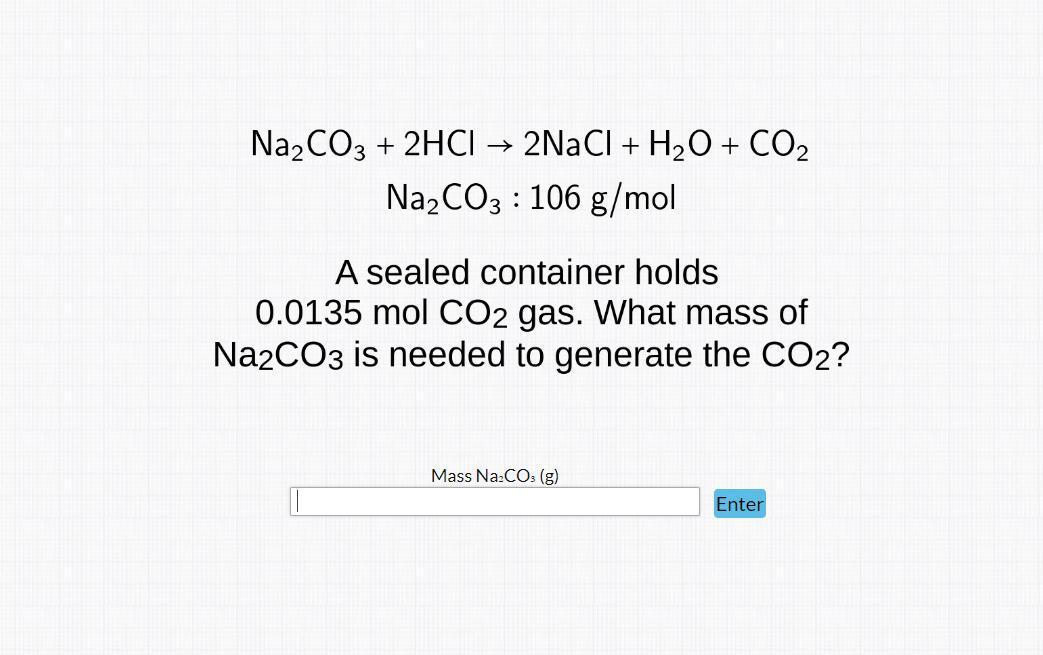
Answers
The mass of Na₂CO₃ needed to generate 0.0135 mole of CO₂ is 1.431 grams
How do I determine the mass of Na₂CO₃ needed?We'll begin by obtaining the mole of Na₂CO₃ that reacted to produce 0.0135 mole of CO₂
Na₂CO₃ + 2HCl -> 2NaCl + H₂O + CO₂
From the balanced equation above,
1 mole of CO₂ was obtained from 1 mole of Na₂CO₃
Therefore,
0.0135 mole of CO₂ will also be obtained from 0.0135 mole of Na₂CO₃
Now, we shall determine the mass of Na₂CO₃ needed for the reation. Details below:
Mole of Na₂CO₃ = 0.0135 moleMolar mass of Na₂CO₃ = 106 g/molMass of Na₂CO₃ = ?Mole = mass / molar mass
0.0135 = Mass of Na₂CO₃ / 106
Cross multiply
Mass of Na₂CO₃ = 0.0135 × 106
Mass of Na₂CO₃ = 1.431 grams
Therefore, the mass of Na₂CO₃ needed is 1.431 grams
Learn more about mass:
https://brainly.com/question/21940152
#SPJ1
Related Questions
Mg+I2=MgI2 Write balanced half-reactions describing the oxidation and reduction that happen in this reaction.
Answers
These are the oxidation and reduction reactions.
What is oxidation?
The process of oxidation occurs when a reactant loses electrons during a reaction. Reduction is the name for the process wherein a reactant picks up electrons while undergoing a reaction. This is a typical event when metals and acids interact. The process of oxidation occurs when a reactant loses electrons during a reaction.
What is reduction?
The process of obtaining electrons or lowering an element's oxidation state in a chemical reaction is known as reduction , which is the transfer of electrons between species. The number of electrons attached to a single atom or a collection of related atoms increases during a chemical reaction known as reduction.
Mg+I2=MgI2
MgI2
Mg is in +2 oxidation state
I2 is in -1 oxidation state
oxidation Mg ⇒ Mg ²⁺ + 2e⁻
Reduction I₂ + 2e⁻ ⇒ 2I⁻
Therefore, these are the oxidation and reduction reactions.
Learn more about oxidation from the given link.
https://brainly.com/question/25886015
#SPJ4
5. A certain radio wave has a wavelength of 7 inches. a. Convert the wavelength of this radio wave into
meters. (1 meter = 39.37 inches) b. Find the frequency of this radio wave.
Answers
Answer: 0.18 (rounded) if you need exact, it's 0.1778
Explanation: I just divided 7 by 39.37 and then checked with g oogle to see if I was doing it right.
but also, that's physics, not chemistry
How many milliliters of 12.0 M HCl(aq) are needed to prepare 545.0 mL of 1.00 M HCl(aq)?
Please answer thank you !
Answers
Answer: 45.42 mL
Explanation: M1V1 = M2V2
A buffer has a pH of 7.7. If a little HCl is added to this buffer, which of the following is the most likely pH value for the mixture?
a) 2.7
b) 5.9
c) 6.1
d) 6.3
e) 7.0
f) 10.2
Answers
pH value for the mixture when HCl is added is 7.
When HCl (strong acid) is added to this buffer system, the extra H+ ions added to the system are consumed by the NH3 to form NH4+. Now, because all the extra H+ ions are locked up and have formed a weaker acid, NH4+, thus the pH of the system does not change significantly.
What is a mixture?
Mixtures can be either homogeneous or heterogeneous: a mixture in which the components are distributed evenly, such as salt in water, is called homogeneous, while a mixture in which the components are clearly separated from each other, such as sand in water, is called heterogeneous.A homogeneous mixture is characterized by the uniform dispersion of the components that make it up; substances exist in the same ratio everywhere in a mixture.To know more about mixture, click the link given below:
https://brainly.com/question/8282825
#SPJ4
Which would you predict to have a higher boiling point and why?
Salt: NaCl
Sugar: C12H1801
Answers
Answer:
salt
Explanation: This is because salt (salt solution) contains more water than sugar. When it boils, the heat of the liquid water and vaporized salt combine to create a hot gas, which pressure forces out from the bottom of the container.
a wastewater is to be dechlorinated using sulfur dioxide. estimate the stoichiometric dose to neutralize a chlorine residual of 6.5 mg/l.
Answers
191.82 [tex]\frac{lbs}{D}[/tex] calculate the stoichiometric dosage to neutralize 6.5 [tex]\frac{mg}{l}[/tex] parts per million of chlorine.
Assume a SO₂ residual of 6.5 [tex]\frac{mg}{l}[/tex] to assure all of the chlorine is reacted.
Then, the feed rate of SO₂ is (6.5 [tex]\frac{mg}{l}[/tex]) + (5.0 [tex]\frac{mg}{l}[/tex]) = 11.5 [tex]\frac{mg}{l}[/tex]
Feed rate in [tex]\frac{lbs}{day}[/tex] = ( 2[tex]\frac{G}{D}[/tex]) × (11.5) × (8.34 [tex]\frac{lbs}{G}[/tex])
Feed rate of SO₂ in [tex]\frac{lbs}{day}[/tex] = 191.82 [tex]\frac{lbs}{D}[/tex]
Simply put, what is stoichiometry?
Stoichiometry, a branch of chemistry, uses relationships between reactants and/or products of a chemical reaction to determine the quantitative data that is required. Since the Greek terms stoikhein and metron both mean element and measure, respectively, stoichiometry literally translates as the measure of elements.
A stoichiometric reaction is what exactly?
When all of the reactants are consumed and none are left behind after the chemical reaction is finished, this is referred to as a stoichiometric chemical reaction. For quantifying chemical reactions, such as those that take place in corrosion processes, stoichiometry is helpful.
To know more about Stoichiometry visit:
https://brainly.com/question/9743981
#SPJ4
100 POINTS HELPPP Which of these is a potential use for a beaker?
A. Obtaining reagents from the original bottle
B. Holding a liquid that evaporates fast at room temperature
C. Creating a specific concentration of a solution
D. Making specific (precise) measurements
Answers
Answer:
B. Holding a liquid that evaporates fast at room temperature
Explanation:
Beakers are useful as a reaction container or to hold liquid or solid samples. They are also used to catch liquids from titrations and filtrates from filtering operations. Laboratory Burners are sources of heat. Burets are for addition of a precise volume of liquid.
B. Holding a liquid that evaporates fast at room temperature
the hydrides of group 5a are nh3 , ph3 , ash3 , and sbh3 . arrange them from highest to lowest boiling point. rank the molecules from highest to lowest boiling point. to rank items as equivalent, overlap them.
Answers
The decreasing order of the hydrides of Group 5A is as follows: SbHN₃ > NH₃ > AsH₃ > PH₃.
What is hydrides?
Any of a group of chemical compounds called hydrides in which hydrogen is joined to another element .
What is boiling point?
A liquid's boiling point is determined by the temperature at which its vapor pressure equals that of the gas above it. The temperature at which a liquid's vapor pressure equals one atmosphere is known as the normal boiling point of that liquid (760 torr).
Therefore, decreasing order of the hydrides of Group 5A is as follows: SbHN₃ > NH₃ > AsH₃ > PH₃.
Learn more about hydrides from the given link.
https://brainly.in/question/7485231
#SPJ4
calculate the molar internal energy of carbon dioxide at 298.15k , taking it's translational and rotational degrees of freedom into consideration
Answers
Answer:
Explanation:
To calculate the molar internal energy of a gas at a given temperature, you need to know the molar specific heat capacities at constant volume and constant pressure for the gas. These values are typically provided in tables of thermodynamic data, which can be found in various sources such as textbooks or online. Since you mentioned that you want to take the translational and rotational degrees of freedom into consideration, you will need to use the molar specific heat capacity at constant volume, which accounts for these degrees of freedom.
Once you have the molar specific heat capacity at constant volume for the gas, you can use the equation U = Cv * T, where U is the molar internal energy, Cv is the molar specific heat capacity at constant volume, and T is the temperature in kelvins. In your case, the temperature is 298.15 K, so plugging in the appropriate values and solving for U will give you the molar internal energy of carbon dioxide at that temperature.
It's important to note that the molar specific heat capacity at constant volume is typically a function of temperature, so you will need to use the appropriate value for the temperature you are interested in. Additionally, different sources may provide slightly different values for the molar specific heat capacity, so it's always a good idea to consult multiple sources to get a sense of the range of possible values.
52.6 g sample of granite initially at 125°C was added to a coffee cup Killorin mentor the calorimeter held 100 mL of water at 20°C what will be the final temperature in the calorimeter
Answers
The final temperature of the granite and the water in the calorimeter is 8.4°.
What is the final temperature?We know that in accordance to the first law of thermodynamics, energy is neither created nor destroyed but it can be converted from one form to another. This implies that the heat that is lost by the granite has to be equal to the heat that is gained by the water in this case.
Knowing that;
H = mcdT
H = heat lost or gained
c = specific heat capacity of the substance
dT = temperature change
Heat lost by the granite = Heat gained by water
52.6 * 0.79 * (θ - 125) = 100 * 4.18 * (θ - 20)
41.6θ - 5200 = 418θ - 8360
Collecting the like terms
41.6θ - 418θ = - 8360 + 5200
-376.4θ = -3160
θ = -3160/-376.4
θ = 8.4°
Learn more about calorimeter:https://brainly.com/question/4802333
#SPJ1
Explain why an object might be classified as having a negative electrostatic charge.
Answers
A negative charge indicates that an item contains more electrons than protons, that's why an object might be classified as having a negative electrostatic charge.
What is negative electrostatic charge?An item has an excess of electrons and is considered to have a negative charge when it obtains electrons. When an item loses electrons, it is considered to have a positive charge because it lacks electrons. Static electricity is the accumulation of electric charges.
Transferring electrons to or taking them away from an item produces an electrical charge. When electrons are introduced to an item, it acquires a negative charge due to the negative charge of electrons.
To know more about electrostatic charge refer to:
https://brainly.com/question/25923373
#SPJ1
Place the following events related to hyperglycemia and vascular injury in the proper order. Reduced blood flowChronic hyperglycemiaEndothelial injuryCalcified plaqueAtherosclerotic plaque2, 3, 1, 4, 53, 2, 1, 4, 52, 3, 4, 5, 12, 3, 5, 4, 13, 2, 1, 5, 4
Answers
2, 3, 5, 4, 1 is the Correct order which is relater to hyperglycemia and vascular injury.
What is Hyperglycemia?
The word "hyperglycemia" comes from the Greek words "hyper" (high) and "glykys" (sweet/sugar") (blood). Blood sugar levels of more than 125 mg/dL when fasting and more than 180 mg/dL two hours after eating are considered to be hyperglycemia. A patient has pre-diabetes or impaired glucose tolerance if their fasting plasma glucose is between 100 and 125 mg/dL. The pathophysiology of hyperglycemia, its presentation, and consequences are covered in detail in this exercise, which also emphasises the need of the interprofessional team in diagnosing and treating patients with this illness.
Untreated hyperglycemia can result in a variety of significant, life-threatening consequences, including harm to the kidneys, heart, eyes, nerves, and peripheral vascular system. Thus, it is essential to properly and efficiently treat hyperglycemia to minimise illness complications and enhance patient outcomes.
Learn more about Hyperglycemia from given link
https://brainly.com/question/23857928
#SPJ4
A sample of nitrogen gas is kept in a container of volume 2.3 L at a temperature of 32 degrees Celsius exerts a pressure of 4.7 atm. Calculate the number of moles of gas present.
Answers
The number of moles of gas present would be 0.43 mol.
Ideal gas equationThe ideal gas law is mathematically expressed as the ideal gas equation and is written as:
pv = nRT
Where p is the pressure, v is the volume, n is the number of moles, R is the gas constant, and T is the temperature of the gas in kelvin.
In this case, the pressure of the gas is given as 4.7 atm, the volume is given as 2.3 L, R is 0.082057 L.atm.K-1.mol-1, and the temperature is given as 32 degree Celsius.
32 degree Celsium = 32 + 273 = 305 kelvin
Making n the subject in the above equation:
n = pv/RT
= 4.7 x 2.3/0.082057x 305
= 10.81/25.027385
= 0.43 mol
In other words, the number of moles of gas present in a sample of nitrogen gas kept in a container of volume 2.3 L at a temperature of 32 degrees Celsius exerts a pressure of 4.7 atm is 0.43 mol.
More on the ideal gas law can be found here: https://brainly.com/question/28257995
#SPJ1
What are the final products (or "outputs") of the Citric Acid Cycle? Select all that apply.
A. Pyruvate
B. Acetyl CoA
C. Citrate
D. NADH
E. CO2
F. ATP
G. FADH2
Answers
The final products (or "outputs") of the metabolic Citric Acid Cycle include NADH and FADH2 (Options D and G are correct).
What is the metabolic Citric Acid Cycle pathway?The metabolic Citric Acid Cycle pathway refers to a step during cellular respiration after glycolysis which has as its main objective the generation of NADH and FADH₂ which serves to transport electrons that will be used during oxidative phosphorylation.
Therefore, with this data, we can see that the metabolic Citric Acid Cycle pathway generates NADH and FADH₂ which are transport electron carriers to generate ATP.
Learn more about the Citric Acid Cycle pathway here:
https://brainly.com/question/29619097
#SPJ1
3. for the reaction 2a b ---> c d (stoichiometric), the following mechanism is proposed: step 1: a b <---> e (forward rate constant
Answers
The rate law expression for the reaction is r = k [A] [B]. Answer B.
The full question is in the attachment. The reaction is bimolecular because it involves two elements as reactants. The two reaction steps present will form the other reaction
A + B → C + D (slow)
A + C → E + (fast)
2A + B → D + E
The rate law for every step
Step 1 : r₁ = k₁ [A] [B]Step 2 : r₂ = k₂ [A] [C]The rate law for the overall reaction depends on the slow step reaction, step number 1
r = k [A] [C]
where k is the rate constant.
Learn more about rate law here: https://brainly.com/question/16981791
#SPJ4
Nitrous oxide can be formed by the thermal decomposition of ammonium nitrate:
NH4NO3(s) ---------> N2O(g) + 2H2O(g)
What mass of ammonium nitrate is required to produce 145 L of N2O at 2850 torr and 42 C?
Answers
The mass of the ammonium nitrate required is equal to 1684 grams.
What is the ideal gas equation?The ideal gas law can be specified as an equation of the state of an ideal gas. This equation is the product of the volume (V) and the pressure (P) is equal to the product of the moles (n), universal gas constant (R), and absolute temperature (T).
The ideal gas equation can be represented as mentioned below:
PV = nRT
Given, the volume of nitrous oxide, V = 145 L
The temperature of nitrous oxide gas, T = 42° C = 42 + 273 = 315 K
The value of the gas constant, R=0.082 atmL/K mol
The pressure of the gas, P = 2850 torr = 3.75 atm
The number of moles of nitrous oxide gas, n = PV/RT
n = 3.75 ×145/(0.082 × 315)
n = 21 mol
The balanced chemical equation:
[tex]NH_4NO_3(s) \longrightarrow N_2O(g) + 2H_2O(g)[/tex]
Therefore, the moles of ammonium nitrate will react = 21 mol
The mass of ammonium nitrate = 21 × 80 = 1684 g
Learn more about ideal gas equation, here:
brainly.com/question/3637553
#SPJ1
With enough ____________,
_______________, and
__________, the original
sedimentary or igneous rock is
changed to ______________ rock.
Answers
Answer: with enough heat, pressure, and time, the original sedimentary or igneous rock is changed to metamorphic rock.
Explanation:
which of the following is not a factor that affects the percentage of light that is transmitted through a colored solution
Answers
Intensity of incoming light is the following is not a factor that affects the percentage of light that is transmitted through a colored solution.
What is intensity?
As well as being the same as the energy density times the wave speed, intensity is the amount of energy a wave transports over a surface of a given area in a unit of time.
What is light?
One kind of electromagnetic radiation is visible light, which travels through space in a way similar to a wave. Gamma rays, X-rays, ultraviolet light, infrared light, microwaves, and radio waves are further forms of electromagnetic radiation.
Therefore, Intensity of incoming light is the following is not a factor that affects the percentage of light that is transmitted through a colored solution.
Learn more about intensity from the given link.
https://brainly.com/question/6544226
#SPJ4
given the following diagram, indicate whether each labeled position is a reactant, a product, a transition state, or an intermediate.
Answers
The 2 transition state and 2 intermediate state are present and finally one product is there.
What is transition state ?
A transition state is an extremely transient configuration of atoms in a reaction-energy diagram that is at a local energy maximum (aka reaction coordinate). An unstable condition known as a transition possesses partial bonding, a very short lifetime (measured in femtoseconds), and cannot be isolated.
What is intermediate state ?
Any species that reacts and is not a transition state, no longer a starting material or reactant, nor a product.
According to the peaked Ness the ranges are named from A to G
A- Reactant
B- transition state
C- intermediate state
D- transition state
E -intermediate state
G- Product
There are 2 transition state and 2 intermediate state are present and finally one product is there.
Learn more about transition state from the given link.
https://brainly.com/question/476481
#SPJ4
8g and 4cm in density
Answers
The density of a piece of wood with the given values for its mass and volume would be = 0.042g/cm³
What is density?Density is defined as the quantity that shows how the particles or molecules of a substance as packed together in a unit mass. It is therefore known as the mass per unit volume of a substance.
Density is very important because in a liquid medium, it allows us to determine what substances will float and what substances will sink.
Mathematically, density can be represented as follows:
Density = mass/volume = m/v, which is measured in kg/m³ or g/cm³.
The volume of the wood = 4× 6× 8 = 192cm³
The mass of the given wood = 8g
The density = 8/192 = 0.042g/cm³
Therefore, the density of the piece of wood which has the following given values would be = 0.042g/cm³.
Learn more about density here:
https://brainly.com/question/28348989
#SPJ1
Complete question:
A rectangular piece of wood measures 4cm by 6cm by 8cm and has a mass of 8g. What is the density of wood of the piece?
Predict whether S for each reaction would be greater than zero, less than zero, or too close to zero to decide.
H2(g) + F2(g)2HF(g)
2NOBr(g)2NO(g) + Br2(g)
2HBr(g) + Cl2(g)2HCl(g) + Br2(g)
4HCl(g) + O2(g)2H2O(g) + 2Cl2(g)
CaCO3(s)CaO(s) + CO2(g)
Answers
Entropy (S) for H₂(g) + F₂(g) ⇒2HF(g) would be less than zero. for 2NOBr(g )⇒2NO(g) + Br₂(g)would be greater than zero. for 2HBr(g) + Cl₂(g) ⇒ 2HCl(g) + Br₂(g) would be too close to zero to decide. for 4HCl(g) + O₂(g) ⇒ 2H₂O(g) + 2Cl₂(g) too close to zero to decide. for CaCO₂(s) ⇒ CaO(s) + CO₂(g)would be greater than zero.
Entropy is typically higher in reactions that entail the transformation of a solid into a liquid or a liquid into a gas.
Entropy is typically lower in reactions involving the transformation of gases into liquids and liquids into solids.
H₂(g) + F₂(g) ⇒2HF(g)Due to the fact that the total number of products generated decreased from 2 gases to 1, there was less degree of disorderliness, making this value less than zero.
2NOBr(g⇒ 2NO(g) + Br₂(g)Due to the formation of two products from one, this is more than zero. The number of gas products increased from one to two, further escalating the system's dysfunction.
2HBr(g) + Cl₂(g) ⇒2HCl(g) + Br₂(g)Due to the reactant and product both having an identical number of products in the same state of matter, this is too near to zero to decide.
4HCl(g) + O₂(g) ⇒2H2O(g) + 2Cl₂(g)Due to the reactant and product both having an identical number of products in the same state of matter, this is too near to zero to decide.
CaCO₃(s) → CaO(s) + CO₂(g)Due to the formation of one gas products from one solid, this is more than zero. The number of gas products increased from zero to one, further escalating the system's dysfunction.
Learn more about entropy at https://brainly.com/question/28224602
#SPJ4
For a radical addition reaction involving HBr and propene, sort each reaction step into the following categories: CH3CH=CH2 + HBr CH3CH2CH2Br
Answers
Answer:
Explanation: Breaking double bond and adding HBr with a catalyst as hydrogen peroxide.
which of the following is a disadvantage of using a lithium ion (li-ion) battery over a nickel metal hydride (nimh) battery?
Answers
The price of lithium ion batteries is a significant drawback. They typically cost about 40% more to produce than nickel-cadmium cells.
What applications does lithium have?Rechargeable batteries for cell phones, computers, digital cameras, and electric vehicles are the most significant applications for lithium. Many non-rechargeable batteries for devices like heart pacemakers, toys, and clocks also include lithium.
Whose lithium deposits are the largest?With 8 million tons, Chile possesses the largest known lithium resources in the world. In comparison, Russia (1.2 million tons), Australasia (2.7 million tons), and Argentina (2 metric tons) are all ahead of the South American country (1 million tons). Compared to the rest of Europe, Portugal has fewer of the expensive raw materials.
To know more about lithium visit:
https://brainly.com/question/1439744
#SPJ4
7. What are the coefficients that will balance the skeleton equation below?
N₂ + H₂ → NH3
a.1,1,2
b.3,1,3,1
c.1,1,1,3
d.1,3,3,1
When the equation Fe + Cl₂ → FeCl, is balanced, what is the coefficient
Answers
Answer:
N₂ + 3H₂ → 2NH3
4Fe + 2Cl₂ → 4FeCl
Explanation:
This equations are now balanced
pls rate as brainliest
A large tire contains 9.5 L of gas at a pressure
of 3.3 atm and a temperature of 279 K.
If the temperature of the gas increases to 303 K
and the volume is increased to 11.9 L,
what will be the new pressure of the gas?
Answers
Answer:
new pressure = 2.86 atm
Explanation:
To solve the given problem, we have to use the 'combined gas law', which is expressed in the formula:
[tex]\boxed{\frac{p_1V_1}{T_1} = \frac{p_2V_2}{T_2}}[/tex].
From the question, we know that the initial volume of the gas is 9.5 L, the initial pressure is 3.3 atm, and the initial temperature is 279 K. Therefore, V₁ = 9.5, p₁ = 3.3, and T₁ = 279.
We are also told that the gas temperature increases to 303 K and the volume increases to 11.9 L. Therefore, T₂ = 303 and V₂ = 11.9. We are then asked to calculate the new pressure (p₂).
To do this, we have to substitute the known values into the equation and solve it for p₂:
[tex]{\frac{p_1V_1}{T_1} = \frac{p_2V_2}{T_2}}[/tex]
⇒ [tex]\frac{3.3 \times 9.5}{279} = \frac{p_2 \times 11.9}{303}[/tex]
⇒ [tex]303 \times \frac{3.3 \times 9.5}{279} = p_2 \times 11.9[/tex]
⇒ [tex]\frac{3.3 \times 9.5 \times 303 }{279 \times 11.9} = p_2[/tex]
⇒ [tex]p_2 = \bf 2.86 \ atm[/tex]
Therefore, the new pressure of the gas is 2.86 atm.
4. Calculate the new molarity if 130.0 mL of water is added to 55.0 mL of a 5.82 M solution of
LIOH.
Answers
The new molarity is 1.73 M
What is the molarity?The new molarity if 130.0 mL of water is added to 55.0 mL of a 5.82 M solution of LIOH.
The formula for determining molarity from moles and volume is extremely straightforward. Simply divide the volume of solution by the moles of solute.The number of moles per liter, denoted by the unit sign mol/L or mol/dm3 in SI units, is the most widely used unit for measuring molarity. One mol/L of a solution's concentration is referred to as one molar, or 1 M.MO = 2.00 M
MD=
MOVO = MDVD
VO = 55 mL, and
VD = 185 mL (55 + 130 mL).
M x 55 mL = 5.82 MD x 185 mL
MD = 5.82*55/185 = 1.73 M
The new molarity is 1.73 M.
To learn more about molarity refer to:
https://brainly.com/question/14469428
#SPJ1
explain why water and solutions with water as the solvent boiled at temperatures lower than 100°c
Answers
Answer:
Below
Explanation:
Water boils at 100°C at sea level under standard atmospheric pressure.
However, the boiling point of a solution, such as a sugar solution, can be lower than the boiling point of pure water.
This is because the presence of solute molecules in the solution reduces the energy needed for the water molecules to escape from the surface of the solution and enter the air as steam.
As a result, the solution will begin to boil at a lower temperature than pure water.
Draw the Lewis structures for the following particles. Which one can exhibit resonance? A. PCI3 B. NO₂¹ C. GeF D. CH₂
Answers
1. The lewis structure of the of phosphorus chloride, PCI₃, nitrogen dioxide, NO₂, germanium fluoride, GeF and methylene, CH₂ are all attached in the image accordingly.
2. The chemical species from above which exhibit resonance from the task above is NO₂
The correct answer choice is option b.
What is meant by lewis structure?Lewis structure can simply be defined as the process which involves the representation of valence electrons shell of an element or a molecule in an electron dot manner.
From the task given above, the lewis structure phosphorus chloride, nitrogen dioxide, germanium oxide and methylene have been correctly identified. All these are chemical compounds whose structure has been represented in an electron dot diagram.
In conclusion, we can now confirm and deduce from the explanation given above that the knowledge of lewis structure helps to understand molecules more.
Read more on lewis structure:
https://brainly.com/question/13820123
#SPJ1
Calculate the mass of hydrogen in your 50.0 mL of water.
Answers
50.0 mL/1000L = 0.05 liters
Then divide by 22.4 for moles
0.05/22.4 = 0.0022 moles of H2O we want hydrogen so for every two moles of hydrogen there’s one water
0.0022 x 2 H/ H2O = 0.0044 moles of hydrogen
Convert to mass by multiplying by its molar mass
0.0044 x 1.01 = 0.0045 grams of Hydrogen is the mass in 50 mL of water
What process is being shown in this diagram?
precipitation
metathesis
dissolution
combustion
Answers
Answer:
dissociation
correct answer mark brainliest
This is the correct answer