50 cm3 of a gas were collected during a reaction. The reaction was carried out at room temperature and pressure. How many moles of the gas were collected? give your answer to 3 decimal places.
Answers
The moles of gas collected during the reaction carried out under normal conditions of pressure and temperature and occupying a volume of 50 cubic centimeters were 0.002 moles.
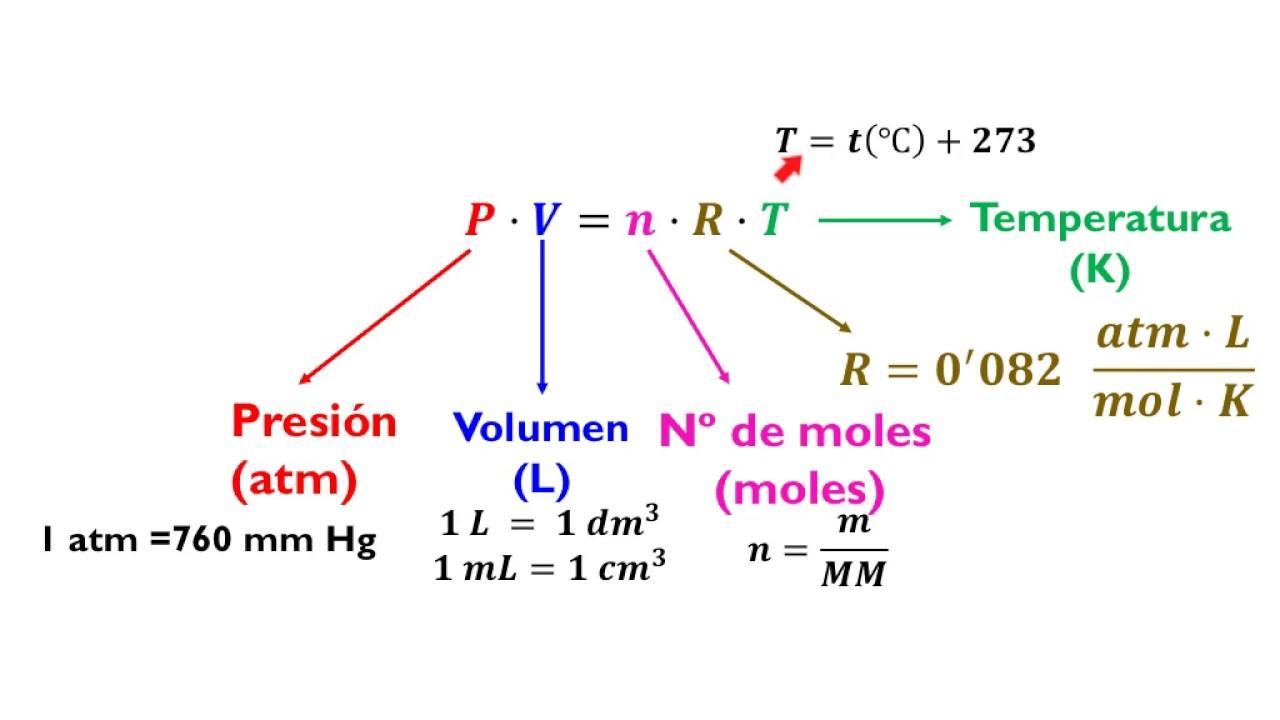
Calculation of moles of gasTo calculate the number of moles of gas that were collected, the ideal gas formula PV = nrt is used
Procedure to calculate the number of moles of gasAssuming the normal conditions of temperature and pressure, we have the following data
n = ?
V = 50 cm3 = 0.05 L
R = 0.082 L atm / mol °K
t = 25°C = 298°K
P = 1 atm
n = PV / Rt
n = 1 x 0.05 / 0.082 x 298
n = 0.05 / 24,436
n = 0.002 mole
Learn more about moles of gas collected during reactions at https://brainly.com/question/1217205
#SPJ4
Related Questions
According to molecular orbital theory, the regions of the wave function with the highest probability of finding electrons are areas with _______.
Answers
According to molecular orbital theory, regions of wave function with highest probability of finding electrons are areas with constructive interference.
An electron is a negatively charged subatomic particle that can exist either free or bound to an atom (not bound). A bound electron is one of the three primary types of particles that make up an atom, along with protons and neutrons. Protons, neutrons, and electrons combined make up the atom's nucleus. A proton's positive charge balances an electron's negative charge. When an atom has an equal number of protons and electrons, it is said to be in a neutral state. Electrons are distinct from other particles in a number of ways. They have a much lower mass, are found outside the nucleus, and exhibit both wave- and particle-like characteristics. The electron is a basic particle.
To know more about electrons visit :https://brainly.com/question/23966811
#SPJ4
The speed of light is about 300,000,000 m/s or 186,000 miles/second. What
parts of the electromagnetic spectrum move at this speed?
Answers
Answer: all of it.
Explanation:
if 14.2 g of al(no3)3 is dissolved in 655g of water, what is the boiling point of the solution (assume 100% dissociation, kb (h2o)
Answers
The boiling point of the solution is 100.208°C.
What is boiling point?The boiling point is defined as elevation in temperature. It is a colligative property means which depends on the concentration of solute.
The temperature at which the pressure of a liquid exerted by its surroundings equals the pressure of the liquid's vapour is known as the boiling point.
At this temperature, the addition of heat causes the liquid to turn into its vapour without raising the temperature.
Molar mass of Al(NO[tex]_3[/tex])₃ = 212.996 g/mol
No. of moles = 14.2/212.996 g/mol
= 0.066 mol
Molarity = 0.066/655 × 1000
= 0.1 M
i = 4 ⇒ ΔT = 0.52° × 0.1 × 4
ΔT = 0.208°
Final temperature = 100° + 0.208°
= 100.208°
For more information about boiling point of solution please visit:
https://brainly.com/question/29233996
#SPJ4
100 POINTS HELP PLEASEEE BRAINLIEST
Answers
Answer:
C
Explanation:
The answer is 10°c
PLEASE MARK AS BRAINLIEST
a sample of gas initially at 0.92 atm and a temperature of 23.2 oc is heated to 40.1 oc. the resulting pressure is 1.3 atm which occupies a volume of 500. ml. what is the initial volume of the sample in milliliters?
Answers
From the gas law, PV=nRT we can say that P(pressure), V(volume) and T (temperature) are related to each other. The initial volume of sample is 408 ml
Given that initial volume of the sample = V1
initial pressure of the sample = 0.92atm
initial temperature of the sample = 23.2oC
Final pressure of the sample = 1.3atm
Final temperature of the sample = 40.1 oC
Final volume of the sample = 500ml = 0.5L
Using the relation P1V1/T1 = P2V2/T2
V1 = 1.3x0.5x23.2/40.1x0.92 = 15.08/36.892 = 0.408
Hence the initial volume of sample = 408 ml
To learn more about pressure click here https://brainly.com/question/12971272
#SPJ4
If the bond angle between two adjacent hybrid orbitals is 109.5°, which is the hybridization?
a. Sp2
b. Sp3d
c. Sp3
d. Sp
Answers
If the bond angle between two adjacent hybrid orbitals is 109.5°, the hybridization is sp³. ore about
The hybridization is sp³ for the bond angle between two adjacent hybrid orbitals is 109.5°. the electrons groups are involved in this process. the four orbitals involves in the sp³ hybridization , the one s orbital and the p orbitals are involve and the hybrid orbital is formed is as sp³ hybridization. the geometry is tetrahedral. the example of the sp³ is the methane.
Thus, for the sp³ hybridization the bond angle between two adjacent hybrid orbitals is 109.5° and the geometry is tetrahedral.
To learn more about hybridization here
https://brainly.com/question/29729198
#SPJ4
if each of these ions were reduced to metal with one coulomb, which would yield the greatest mass? (a) cu2 (aq)(b) ag (aq) (c) hg2 (aq) (d) cu (aq)
Answers
The option B is correct. Ag will yield the greatest mass
Faraday proposed two electrolysis laws. The mass of an element released during electrolysis is directly proportional to the amount of electricity flowing through the electrolyte, according to the first law. Mathematically, this can be expressed as:
Mass deposited, (M) ∝ current, (I) × time, (t)
or M ∝ It.
According to the second law, the masses of different elements freed when a constant amount of power is passed through distinct electrolytes are inversely related to the chemical equivalents of the ions undergoing reaction. Making this into an equation:
″Mass deposited, M ∝ E where
E= mass of 1 mole of an ion with a charge of + 1,
So, we can see that out of all the options given Ag has the greatest value of E. So it will be the correct option.
To know more about electrolysis, please refer:
https://brainly.com/question/12994141
#SPJ4
What is the most possible effect of overexposure to chemicals?
Answers
The most possible effect of over exposure to chemicals is the weakening of immune system.
There are certain chemicals which are very harmful for the human body such as insecticides and pesticides etc.
Along with them many chemicals which occurs in gaseous state at a room temperature are also very dangerous for human health. For performing research and development procedures the exposure to these Chemicals is not that much considerable.
But when the human body is overexposed to this chemicals, the weakening in the immunity system is observed within the human body because of the contamination of the body due to these chemicals.
To know more about overexposure to chemicals, visit,
https://brainly.com/question/26101660
#SPJ4
the society of automotive engineers has established an accepted numerical scale to measure the viscosity of motor oil. for example, sae 40 motor oil has a higher viscosity than an sae 10 oil. rank the following hydrocarbons by their expected viscosity.a. CH3CH2CH2CH2CH3b. CH3CH2(CH2)6CH2CH3c. CH3CH2(CH2)11CH2CH31. most viscous2. least viscous
Answers
The ranking of the given organic compounds according to the decreasing order of viscosity is CH₃CH(CH₂)₁₁CH₂CH₃ > CH₃CH₂(CH₂)₆CH₂CH₃ > CH₃CH₂CH₂CH₂CH₃, and the increasing order of viscosity is CH₃CH₂CH₂CH₂CH₃ < CH₃CH₂(CH₂)₆CH₂CH₃ < CH₃CH(CH₂)₁₁CH₂CH₃.
Viscosity is a property of material which describes the resistance of a fluid to shearing flows. Viscosity corresponds roughly to the intuitive notion of a fluid's thickness. For example, honey has a much higher viscosity than water.
Viscosity is measured by using a device known as viscometer. Measured values of viscosity span several orders of magnitude. Among all the fluids, gases have the lowest viscosities, and thick liquids have the highest.
The above mentioned hydrocarbon has different viscosity and hence they are arranged in order of their viscosity.
Learn more about viscosity from the link given below.
https://brainly.com/question/2286110
#SPJ4
in the laboratory you dilute 2.29 ml of a concentrated 6.00 m hydrochloric acid solution to a total volume of 100 ml. what is the concentration of the dilute solution?
Answers
The concentration of the diluted solution will be 0.13 M
It asks you to find the concentration, which is the safe to assume that the unit is Molarity (M). Also, the unit was used in the problem as well.
Molarity has the units of moles/L so we have to work on getting to this point.
The problem states that you have the 2.29 mL of a 6.00 M concentration. First, we will need moles. To get the moles, we use stoichiometry,
2.29 mL = 0.00229 L
0.00229 L x ( 6 moles/ L) = 0.01374 moles
0.00229 L x ( 6 moles/ L) = 0.01374 moles
This is how many moles are present inside the solution.
To find the new concentration, we divide the total number of moles by the new volume in the Liters:
0.01374 moles/0.100L = 0.1374 M
Rounding to two significant figures, we get 0.13 M.
To know more about molarity, please refer:
https://brainly.com/question/26873446
#SPJ4
silver nitrate reacts with magnesium chloride to produce silver chloride and magnesium nitrate. if 305 grams of silver nitrate are reacted in an excess of magnesium chloride producing 23.7 grams of magnesium nitrate, what is the percent yield?
Answers
The percent yield of the solution is 17.7% that can be calculated by using experimental and theoretical yield.
Moles of silver nitrate = 305/169.87 = 1.8 moles.
Two moles of AgNO₃ will give 0.5 mole of MgNO₃
Therefore the 1.8 moles of AgNO₃ and excess of MgCl will give 0.9 mole of MgNO₃.
Percent yield can be calculated by dividing the actual yield by the theoretical yield multiplied by 100.
Theoretical yield = 0.9 x 148.3= 133.47
= Yield % = (experimental yield / theoretical yield ) x100
= (23.7/133.47) x 100
= 17.7%
The percent yield of the solution is 17.7%.
To learn more about percent yield check the link below:
https://brainly.com/question/25996347
#SPJ4
If there are 4 H atoms on the reactant side, how many H atoms will be on the product side?
Answers
Answer:-On the reactant side, there are two H atoms and two O atoms; on the product side, there are two H atoms and only one oxygen atom. The equation is not balanced because the number of oxygen atoms on each side is not the same (Figure 7.3. 2). Figure 7.3.
The simplest hydrocarbon contains one carbon atom and four hydrogen atoms. The formula is CH4, and the name is methane.
Steps for Solving for a Reactant in Solution
Step 1: Determine what information is given in the problem and what information you are looking for.
Step 2: Set up unit conversions to find the information you are looking for.
Step 3: Do the calculations for the unit conversions and find the solution.
The equation is fairly simple. The number of atoms of ANY substance in a volume is: # of atoms = N * (density) * volume / (Molecular Weight). N is a constant called Avogadro's number and its equal to 6.022*1023 atoms/mole. Find the number of lone pairs on the central atom by subtracting the number of valence electrons on bonded atoms (Step 2) from the total number of valence electrons (Step 1). Divide the number of VEs not in bonds (from Step 3) by 2 to find the number of LPs.
Which of these ions is more abundant in the interior of resting neuron that in the fluid surrounding the neuron?
A. Cl-
B. Ca++
C. Na+
D. K+
Answers
K+ ions are more abundant in the interior of resting neuron that in the fluid surrounding the neuron.
An active pump can keep the larger concentration of Na+ outside the neuron because there is resistance to the passage of Na+ through the neuronal membrane. At rest, K+ concentrations are higher inside the neuron.
Which ions are more abundant outside of a resting cell?
The sodium ion concentration inside the cell is low, whereas the sodium ion concentration outside the cell is higher.
What ion contributes the most to the resting potential of neurons?
Potassium is the main ion responsible for determining the resting membrane potential. In skeletal muscle, potassium conductance makes up around 20% of the resting membrane conductance, and it makes up the majority of the resting conductance in neurons and nerve fibers.
To know more about neurons visit
brainly.com/question/29462317
#SPJ4
what is the molality of a solution that is prepared by dissolving 12 g of nacl in 100 g of solution? enter your answer to three significant figures and in units of molality.
Answers
The molarity of the solution is 2.05 mol/kg, it can be expressed in terms of kilograms as well as liters.
Molarity is defined as the number of moles of the solute to the volume of the solution in liters or weight of solution in kilograms.
It is expressed as follows:
M= n/V or M=n/W
Molar mass of NaCl = 58.4 g/mol
Given mass of NaCl=12 g
The weight of the solution=100 g
Molarity can be calculated as follows:
M= 12/58.4*0.1
M=2.05 mol/kg
The molarity of the solution is 2.05 mol/kg.
To learn more about molarity check the link below:
https://brainly.com/question/26873446
#SPJ4
What is the attraction an atom has for the shared electrons in a covalent bond called?
Answers
The attraction an atom has for the shared electrons in a covalent bond called is electronegativity.
The electronegativity of the atom is the tendency of the atom to attract the share pair of electrons towards itself. the covalent bond is the bond formed by the transfer of the electrons between the atoms. if the difference in the electronegativity of the atom is higher then it will leads to the complete transfer of the electron and then the ionic bond will form and the compound is called as the ionic compound.
Thus, the electronegativity is the attraction of an atom has for the shared electrons.
To learn more electronegativity here
https://brainly.com/question/17762711
#SPJ4
select the intermolecular force that is overcome when hexane is converted from a liquid to a gas.
Answers
Electrostatic in nature, intermolecular forces result from the interaction of positively and negatively charged entities. Intermolecular interactions are the total of both attracting and repulsive elements, similar to covalent and ionic connections.
What characteristics do intermolecular forces have?
forces of attraction between molecules
Although much less than intramolecular forces of attraction, intermolecular forces play a crucial role in determining the physical characteristics of molecules, including their melting and boiling points, density, and enthalpies of fusion and vaporization.
What determines whether a reaction is intermolecular?
It can be helpful to distinguish between intramolecular and intermolecular processes. While two or more reaction sites within the same molecule are engaged in intramolecular reactions, covalency changes occur in two distinct molecules during intermolecular reactions.
To know more about intermolecular force visit;
https://brainly.com/question/9007693
#SPJ4
Does anyone know what's the incomplete combustion of C2H6?
Answers
Answer:
The incomplete combustion of C2H6, also known as ethane, produces a mixture of carbon monoxide (CO) and hydrogen (H2) gases. This reaction typically occurs when there is not enough oxygen present to support complete combustion, which would produce carbon dioxide (CO2) and water (H2O) instead.
the lattice energy for naoh is -887 kj/mol and this is the energy given off (a negative number) when this lattice forms. we are interested in the opposite process, when the lattice is disrupted. the lattice energy for naoh is -887 kj/mol. calculate the energy required to separate the ions in 5.00 grams of naoh (mw
Answers
110.9KJ is the energy required to separate the ions in 5.0grams of NAoH. For the lattice energy for naoh is -887 kj/mol and this is the energy given off (a negative number) when this lattice forms.
Lattice energy is a measure of the strength of the ionic bonds in an ionic compound. It provides insight into several properties of ionic solids including their volatility, their solubility, and their hardness. The lattice energy of an ionic solid cannot be measured directly. The term ‘ion’ can be used to refer to atoms or molecules that have non-zero net charges associated with them. Therefore, all ions have either a greater number of protons than electrons in their overall atomic or molecular structure.
Learn more about lattice energy here
https://brainly.com/question/18222315
#SPJ4
The energy needed to separate the ions in 5.0 grams of NaOH is 110.9 KJ. The energy released when this lattice develops, which is a negative quantity, is called the lattice energy for NaOH, which is -887 kJ/mol.
The strength of the ionic bonds in an ionic compound is gauged by lattice energy. It sheds light on a number of ionic solids' characteristics, such as their solubility, hardness, and volatility. An ionic solid's lattice energy cannot be directly measured. Atoms or molecules having associated net charges greater than zero are referred to as "ions." As a result, the overall atomic or molecular structure of all ions has more protons than electrons.
Learn more about lattice energy:
https://brainly.in/question/3919074
#AAA
an unknown radioactive substance has a half-life of 3.20 hours . if 30.1 g of the substance is currently present, what mass a0 was present 8.00 hours ago? express your answer with the appropriate units.
Answers
The mass a0 was present 8.00 hours ago is 177.9 grams.
Radionuclides or radioactive substances) are a class of chemical substances where the nucleus of the atom is volatile. They gain stability thru changes in the nucleus (spontaneous fission, emission of alpha debris, or conversion of neutrons to protons or the opposite).
Radioactive sources are used to study residing organisms, to diagnose and treat sicknesses, to sterilize clinical devices and meals, to produce energy for heat and electric powered power, and to screen various steps in all types of business approaches.
Calculation:-
t1/2 = 3.2 hr
k = 0.693/3.2 = 0.216 hr-1.
k= (1/t)ln(a/x)
0.216 = (1/8)ln(a/31.6)
a = initial mass = 177.9 grams
Learn more about radioactive substance here:-https://brainly.com/question/25750315
#SPJ4
If the temperature of a fixed amount of an ideal gas is increased, it necessarily follows that: ________
Answers
If the temperature of a fixed amount of an ideal gas is increased, it necessarily follows that: the average speed of the gas molecules will increase.
What is an ideal gas?Multiple randomly moving point particles with no interparticle interactions make create an ideal gas in a theory. The ideal gas notion is useful because it complies with the ideal gas law, has condensed equation of state, and can be studied using statistical mechanics. If, for an instance, the interaction is totally elastic or is thought of as point-like collisions, the constraint of zero interaction can frequently be disregarded.
Many real gases behave qualitatively like an ideal gas under varied temperature and pressure settings, where the gas molecules (or atoms for monatomic gases) take the place of the ideal particles.
If specific properties are preserved within appropriate bounds over a wide range of temperatures and pressures, many gases, including nitrogen, oxygen, hydrogen, noble gases, some heavier gases like carbon dioxide, and mixtures like air, can be categorized as ideal gases. At higher temperatures and lower pressures, a gas typically behaves more like an ideal gas because the potential energy from intermolecular forces is less significant compared to the kinetic energy of the particles and the size of the molecules is less significant compared to the void space between them.
To learn more about ideal gas visit:
https://brainly.com/question/28257995
#SPJ4
what is the simplest formula of a solid containing a, b, and c atoms in a cubic lattice in which the a atoms occupy the corners, the b atoms the body-center position, and the c atoms the faces of the unit cell?
Answers
The formula for a solid with atoms a, b, and c is abc3, where an atoms are located in the corners, b atoms are in the body-center position, and c atoms are on the faces of the unit cell.
The lattice points are only present in the corners of a simple cubic unit cell, making it the most basic repetitive unit cell.
Since there are eight corners, each carrying an an atom, a atoms = 8x1/8 = 1 atom.
Because there is just one central atom, for b atoms = 1x1 = 1.
There are six sides, each of which contains a c atom, therefore for c atoms = 6x1/2 = 3 atoms.
The simplest formula is abc3
To learn more about lattice click here https://brainly.com/question/18222315
#SPJ4
rrange these compounds from fastest Sg2 reaction rate to slowest Sn2 reaction rate.
bromomethane 1-bromo-3-methylbutane 2-bromo-2-methylbutane 2-bromo-3-methylbutane
Answers
These compounds from the fastest SN2 reaction rate to the slowest SN2 reaction rate will be: Bromomethane > 1-Bromo-3-methylbutane > 2-Bromo-3-methylbutane > 2-Bromo-2-methylbutane.
The SN2 reaction is a Nucleophilic Substitution reaction in which the rate-determining step involves 2 components. Nucleophiles, typically, have a lone pair of electrons in them. They are attracted to the nucleus of an atom. In SN2 reaction an electron-rich nucleophile displaces the halogen atom bonded to the central carbon of an alkyl halide molecule. Some characteristics of SN2 reaction are: it is a one-step reaction process.
Rate of reaction depends on the concentrations of the substrate (alkyl halide) and the nucleophile.
Learn more about SN2 reaction here:
https://brainly.com/question/17031676
#SPJ4
when 0.434 g of sodium metal is added to an excess of hydrochloric acid, 4510 j of heat are produced. what is the enthalpy of the reaction as written?
Answers
The enthalpy of reaction is -238.62 Kj/ mol.
What is enthalpy of reaction?
Enthalpy of reaction (also known as the heat of reaction) is the amount of heat released or absorbed during a chemical reaction. It is an important physical property of a reaction, and is a measure of the reaction’s ability to do work. It is related to the change in internal energy of a reaction and can be calculated using Hess’s law. Enthalpy of reaction can be used to calculate the temperature of the reaction, the rate of reaction, and the amount of energy released or absorbed in the reaction. It is also used to determine the equilibrium constants for a reaction, and can be used to predict the stability of a reaction. Enthalpy of reaction is an important concept in thermodynamics and can be used to study the energetics of a reaction.
The enthalpy per mole of reactant is expressed in units of kj/mol.
(0.434 g of Na) × (1 mole Na / 22.990g) = 0.0189 mole
ΔH= -4.510kj /0.0189 mole = - 238.62 Kj/ mol
ΔH is negative because energy is released by the system.
For more information about enthalpy please visit: https://brainly.com/question/29888663
#SPJ4
A major component of gasoline is octane. When liquid octane is burned in air it reacts with oxygen gas to produce carbon dioxide gas and water vapor. Calculate the moles of oxygen needed to produce of water. Be sure your answer has a unit symbol, if necessary, and round it to significant digits.
Answers
The moles of oxygen needed to produce water is 0.50 mol O2.
When liquid octane is burned in the air, it undergoes a chemical reaction with oxygen gas. Octane is a hydrocarbon, meaning it contains hydrogen and carbon atoms. During the combustion reaction, the hydrogen atoms in the octane react with oxygen molecules (O2) from the air to form water vapor (H2O).
The carbon atoms in the octane react with oxygen molecules (O2) from the air to form carbon dioxide (CO2). This reaction releases energy in the form of heat and light.
The equation for this reaction is as follows:
C8H18(l) + O2(g) → CO2(g) + H2O(g)
For every 1 mole of octane (C8H18) that is burned, 2 moles of oxygen (O2) are needed to produce 1 mole of water (H2O).
Therefore, if we want to calculate the moles of oxygen needed to produce 1 mole of water, we need to divide 1 mole of water by 2 moles of oxygen, which gives us 0.50 moles of oxygen.
The final answer is 0.50 mol O2, which should be rounded to two significant digits.
For more questions like Oxygen moles click the link below:
https://brainly.com/question/13456135
#SPJ4
a newspaper published an article about a study in which researchers subjected laboratory gloves to stress. among vinyl gloves, % leaked viruses. among latex gloves, % leaked viruses. using the accompanying display of the technology results, and using a significance level, test the claim that vinyl gloves have a greater virus leak rate than latex gloves. let vinyl gloves be population 1.
Answers
The null hypothesis is rejected since the Z-value is higher than the crucial value. The prevalence of vinyl gloves which leak viruses is higher than the prevalence of latex gloves which leak viruses.
How is a virus defined?A length of nucleotides (either DNA or RNA), encased in a protein coat, makes up a virus, an infectious bacterium. Since viruses are unable to multiply on their own, they must infect host cells in order to utilise those cells' components as building blocks for their own replication.
What makes something a virus?virus, an infectious agent with a small size and straightforward chemical makeup that can reproduce exclusively in live cells of bacteria, plants, or animals. The word for "poison" or "slimy liquid" in the name is Latin in origin. The first proof of the biological nature of viruses was provided by research done in 1892 either by Russian scientist Dmitry
To know more about virus visit:
https://brainly.com/question/29156932
#SPJ4
a 20.00 ml volume of h2so4 solution is titrated with 0.125 m naoh. the initial volume of naoh in the buret is 0.75 ml and the final volume is 42.35 ml. determine the concentration of the h2so4 solution. a. 0.115 m h2so4 b. 0.150 m h2so4 c. 0.300 m h2so4 d. 0.130 m h2so4 e. 0.260 m h2so4
Answers
The molarity of H2SO4 when a 20.00 ml volume of h2so4 solution is titrated with 0.125 m NaOH. the initial volume of NaOH in the buret is 0.75 ml and the final volume is 42.35 ml is 0.260 M.
The balanced equation is 2NaOH+H2SO4→Na2SO4+2H2O.
The formula to calculate molarity is
c=n/v
n= c×v
Where, n = moles v= volume and c= molarity
So, the moles in NaOH
nNaOH = 0.125 × 0.75/ 1000
= 9.3 × 10^-3
From the equation we can see that number of moles of H2SO4 must be half that amount so:
nH2SO4 = 9.3 × 10^-3 /2
=4.65 × 10^-3
c = n/v
[H2SO4] = 4.65 × 10^-3 /20× 10^-3
= 0.235 mol/L which equivalent to 0.26 M H2SO4
For more information on molarity kindly visit to
https://brainly.com/question/8732513
#SPJ4
How many dna molecules would there be after four rounds of pcr if the initial reaction mixture contained two molecules?
Answers
The number of DNA ( Deoxyribonucleic acid) molecules that are produced after 'n' number of pcr ( polymerase chain reaction) can be given by the formula: N = d x 2ⁿ
where,
'N' is the number of DNA molecules obtained after 'n' number of polymerase chain reactions.
'd' is the number of DNA molecules originally present before the start of pcr cycle.
In the given question,
d = 2;
n = 4;
Then, N = 2 x 2⁴
N = 2 x 16
N = 32;
Thus, the number of DNA molecules that are obtained after 4 rounds of pcr when 2 molecules are present before the start of the process are 32.
Learn more about DNA and PCR at:
brainly.com/question/28444229
#SPJ4
How many ml of .50 M NaOH are required to completely titration 15.0 mL of .20 M HNO_3 solution
Answers
150ml of 0.50 M NaOH are required to completely titration, 15.0 mL of .20 M HNO₃ solution.
What is titration ?To determine the concentration of an identified analyte, titration is a typical laboratory technique for quantitative chemical analysis. A standard solution with a known concentration and volume is prepared as the reagent, also known as the titrant or titrator.
The equivalence point, or the point at which chemically equivalent amounts of the reactants have been combined, is to be detected by the titration. The stoichiometry of the reaction determines how many reactants have been combined at the equivalence point.
Thus, 150ml of 0.50 M NaOH are required to completely titration, 15.0 mL of .20 M HNO₃ solution.
To learn more about titration, follow the link;
https://brainly.com/question/2728613
#SPJ1
how are monomers and polymers related? group of answer choices a monomer is a small, repeating unit that makes up a polymer. a monomer is one strand of a polymer. a monomer is made when you condense a polymer through polymerization. a polymer is chemically identical to a monomer, only larger. none of these
Answers
The sole difference between a polymer and a monomer is size. A polymer is made up of tiny, repeating units called monomers. When a polymer is condensed by polymerization, a monomer is created.
The term "monomer" refers to a single atom, tiny molecule, or molecular fragment that, when joined with other monomers of the same or closely related types, produces a bigger, macromolecule known as a polymer. Through a process known as polymerization, monomers join together to form polymers through the creation of chemical bonds or supramolecular binding. Polymers are huge molecules that are created by the bonding of monomers, which are the components of a molecule. The major categories of macromolecules are known as polymers, which are macromolecules created by the union of monomers that are present in the cell such as proteins, lipids, carbohydrates, and nucleic acids
To learn more about polymers click here https://brainly.com/question/14600435
#SPJ4
) determine the weight-average molecular weight of the polymer, and (b) the mean-squared radius of gyration, r2 g in both solvents. (c) is r2 g the same for both polymers? why or why not? (d) if you were to assume this polymer is polystyrene with a kuhn length of b
Answers
By adding up the weights of each chain and dividing by the total number of chains, the average molecular weight can be calculated.
The average molecular weight is a crucial way to describe polymers. The entire weight of the polymer divided by the total number of molecules yields the average molecular weight. chromatography using gel permeation (GPC) Size exclusion chromatography is another name for gel permeation chromatography. It is a common technique for figuring out high molecular weight distribution. Using this method, chemicals are separated based on the size of their molecules. This is also the root-mean-square distance of the molecule's segments from the center of mass for polymer chains. One indicator of the magnitude of the random coil shape that many synthetic polymers adopt in solution or in the amorphous bulk state is the radius of gyration.
To learn more about polymers click here https://brainly.com/question/825578
#SPJ4