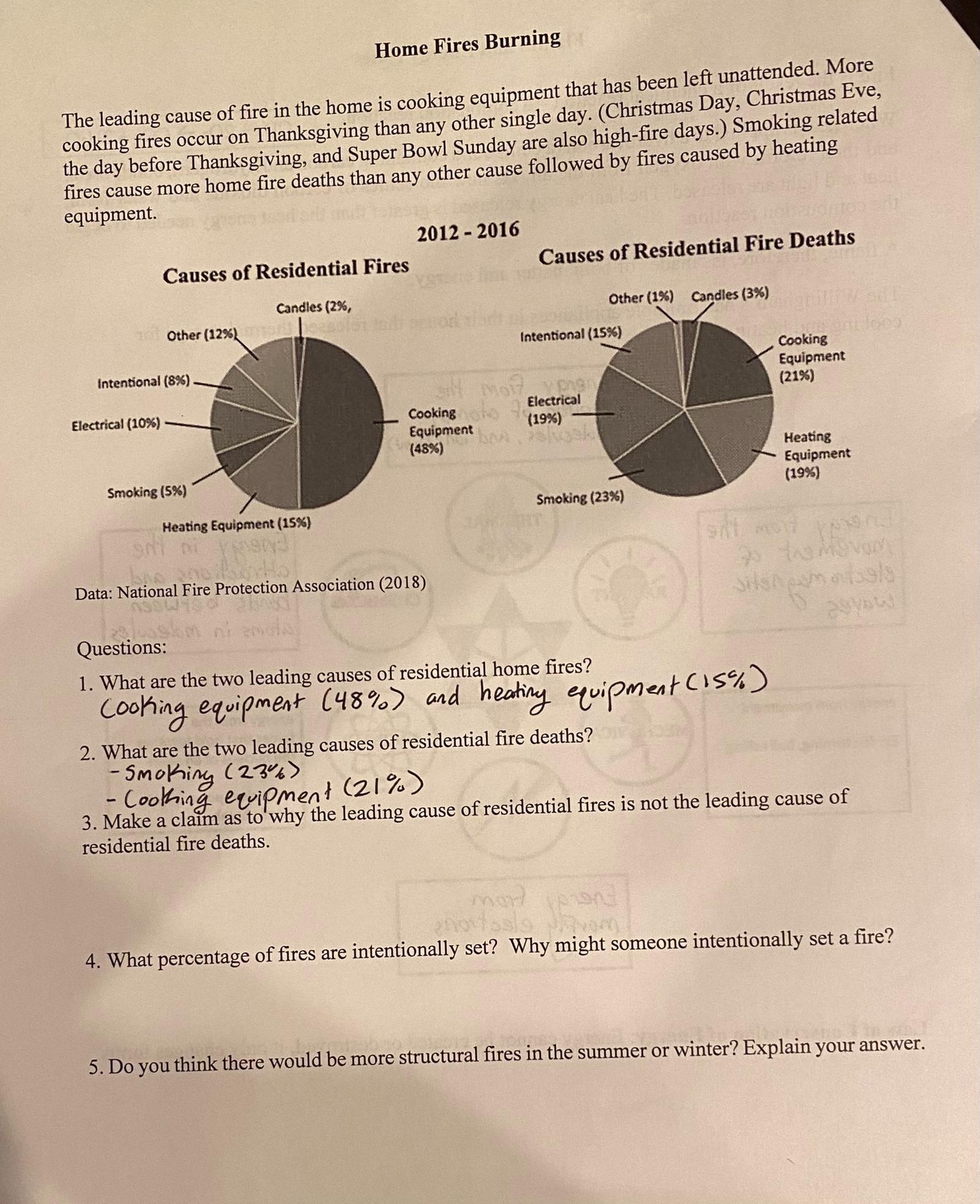
3. Make a claim as to why the leading cause of residential fires is not the leading cause of residential fire deaths.4. What percentage of fires are intentionally set? Why might someone intentionally set a fire?5. Do you think there would be more structural fires in the summer or winter? Explain your answer.
Answers
3. The leading cause of residencial fires (cooking equipment) is not the leading cause of residential death because the fire in the equipment can be extinguished in time, while the effects of smoking can remain unsigned in time.
4. The percentage of intentionally set fires is 8%. Someone might intentionally set a fire for example by burning yard trimmings.
5. There would be more structural fires in summer due to the high temperatures and the lack of rain.
Related Questions
Maria is an 18 year old girl whose parathyroid has stopped functioning properly. What does this mean for the calcium levels in her
body?
Answers
Maria is an 18 year old girl whose parathyroid has stopped functioning properly, this mean calcium levels in her body decreased.
Calcium (Ca) is a chemical element and an alkaline-earth metal from Periodic Table Group 2 (IIa). It is the fifth most abundant element in the Earth's crust and the most prevalent metallic element in human tissue.
The ancients made considerable use of lime (calcium oxide, CaO), a calcium compound. Sir Humphry Davy first extracted the lightweight, silvery metal itself in 1808 after distilling mercury from an amalgam created by electrolyzing a combination of lime and mercuric oxide. The Latin word for lime, calx, served as the inspiration for the element's name. The cosmic abundance of calcium is thought to be 4.9 104 atoms, and it makes up 3.64 percent of the crust of the Earth and 8 percent of the crust of the Moon.
To know more about calcium visit : https://brainly.com/question/3569638
#SPJ9
why do engineers come up with as many ideas to solve a problem as possible?
Answers
Engineers come up with as many ideas to solve a problem as possible is so as to choose the best which is applicable in helping people.
Who is an Engineer?This is referred to as an individual or a professional which employs the use of scientific knowledge in the construction and building of different types of machines and tools.
An engineer is someone who has a good analytic and problem solving skills and comes up with many ideas so as to be able to choose the best from the set of alternatives in helping people solve their problems or issues.
Read more about Engineer here https://brainly.com/question/17169621
#SPJ1
Aluminum bromide can be prepared by reacting small pieces of aluminum foil with liquid bromine at room temperature. The balanced chemical reaction is:2Al(s) + 3Br2(l) → 2AlBr3(s)How many moles of Br2 are needed to produce 5 mol of AlBr3, if sufficient Al is present?
Answers
Based on the mole ratio from the chemical reaction 3 moles of Br2 produces 2 moles of AlBr3. We can set this equation up to determine the unknown:
[tex]\begin{gathered} \frac{2}{3}=\frac{x}{5} \\ 3x=2\times5 \\ x=\frac{10}{3} \\ x=3.3moles \end{gathered}[/tex]3.3 moles of Br2 is needed to reacts with 5 mol of AlBr3.
10.The drink mix and water in a drink solution can be separated by...Select one:a. filtering out the drink mix from the water.b. evaporating the water.c. picking out the drink crystals by hand.d. drawing the drink crystals out with a magnet.
Answers
Answer
b. evaporating the water.
Explanation
The ingredients in a solution cannot be separated by hand because of changes in the ingredients' physical properties. But evaporation can be used to separate some solutions. For example, just in the case of the drink mix and water in a drink solution given.
Identify the equation with the elements in BeSO4 in their standard state as the reactants and BeSO4 as the product.
Answers
Answer:
[tex]E[/tex]Explanation:
Here, we want to identify the elements in BeSO4 in their standard state as the reactants and BeSO4 as the product
From what we have, there are 4 atoms of oxygen, 1 atom of Beryllium, and 1 atom of sulfur
In their standard state, oxygen is a gas, Berrylium and Sulfur are solids
The number of elements atom on the reactant side must be equal to that on the product side
Thus, we have the correct choice of answer as E
You have 6.0 g of C2H6 and 20.0 g of O2 for a combustion reaction. If you actuallyproduce 3.80 g of CO2 , What is the percent yield?
Answers
Answer:
The percent yield is 24.2%.
Explanation:
1st) It is necessary to balance the chemical reaction:
[tex]2C_2H_6+7O_2\rightarrow4CO_2+6H_2O[/tex]2nd) From the balanced reaction, we know that 2 moles of C2H6 react with 7 moles of O2 to produce 4 moles of CO2. With the molar mass of C2H6 (30g/mol), O2 (32g/mol) and CO2 (44g/mol), we can convert the moles to grams:
- C2H6 conversion:
[tex]2moles*\frac{30g}{1mole}=60g[/tex]- O2 conversion:
[tex]7moles*\frac{32g}{1mole}=224g[/tex]-CO2 conversion:
[tex]4moles*\frac{44g}{1mole}=176g[/tex]Now we know that 60g of C2H6 react with 224g of O2 to produce 176g of CO2.
3rd) From the given values of C2H6 (6.0g) and O2 (20.0g), it is necessary to find out which one is the limiting reactant and which one is the excess reactant:
[tex]\begin{gathered} 60gC_2H_6-224gO_2 \\ 6.0gC_2H_6-x=\frac{6.0gC_2H_6*224gO_2}{60gC_2H_6} \\ x=22.4gO_2 \end{gathered}[/tex]We can see that the 6.0g of C2H6 will need 22.4g of O2 to react, but we only have 20.0g of O2, so O2 is the limiting reactant and C2H6 will be the excess reactant.
4th) Now, using the limiting reactant, we have to calculate the grams of CO2 that should be produced from the stoichiometry of the reaction (this is the Theoretical yield):
[tex]\begin{gathered} 224gO_2-176gCO_2 \\ 20.0gO_2-x=\frac{20.0gO_2*176gCO_2}{224gO_2} \\ x=15.7gCO_2 \end{gathered}[/tex]5th) Finally, we can calculate the Percent yield of the reaction, by using the Theoretical yield (15.7g) and the Actual yield (3.80g):
[tex]\begin{gathered} PercentYield=\frac{ActualYield}{TheoreticalYield}*100\% \\ PercentY\imaginaryI eld=\frac{3.80g}{15.7g}*100\operatorname{\%} \\ PercentY\mathrm{i}eld=24.2\% \end{gathered}[/tex]So, the percent yield is 24.2%.
Use the equation: 3H2, + N2 —-2NH3, (all are gasses)If you need to make 467L of ammonia gas, how many grams of nitrogen gás do you need to start with?
Answers
1) First, let's rewrite the equation:
3 H2 + N2 ---> 2 NH3
If you need to make 467 L ammonia gas (NH3), let's find out how many moles of it do we need.
For this, we use the molar volume of gases: 22.4L/mol
So:
467 L ------ x mol
22.4 L ----- 1 mol
22.4x = 467
x = 20.8 mol of NH3
2) Now we use the proportion of the chemical equation to find out the quantity in moles of N2 (nitrogen gas):
1 mol of N2 ---- 2 mol of NH3
x mol of N2 ----- 20.8 mol of NH3
2x = 20.8
x = 10.4 mol of N2
3) Now we use the molar mass of N2 to find the quantity in grams that we need to start with:
molar mass N2 = 2x14 = 28 g/mol
Now we use the following equation:
mass = mole × molar mass
mass = 10.4 × 28 = 291.2 grams of Nitrogen Gas
Answer: 291.2 grams of Nitrogen Gas
What physical property can help characterize fragments of glass at a crime scene?
A.
flammability
B.
toxicity
C.
density
D.
volume
Answers
Answer:
I believe it is
C. density
Explanation:
have a nice day!❀
Calculate the molar mass of NaHCO3 and round it to 2 decimal places.
Answers
To calculate the molar mass of NaHCO₃, we need to add the molar mass of each of its atoms.
This compound has 1 atom of Na, 1 of H 1 of C and 3 of O.
The molar mass of these atoms can be consulted on a periodic table, and they are:
[tex]\begin{gathered} M_{Na}=22.9898g/mol \\ M_H=1.0079g/mol \\ M_C=12.0107g/mol \\ M_O=15.9994g/mol \end{gathered}[/tex]So, the molar mass of NaHCO₃ is:
[tex]\begin{gathered} M_{NaHCO_3}=1\cdot M_{Na}+1\cdot M_H+1\cdot M_C+3\cdot M_O \\ M_{NaHCO_3}=(1\cdot22.9898+1\cdot1.0079+1\cdot12.0107+3\cdot15.9994)g/mol \\ M_{\mleft\{NaHCO_3\mright\}}=\mleft(22.9898+1.0079+12.0107+47.9982\mright)g/mol \\ M_{\mleft\{NaHCO_3\mright\}}=84.0066g/mol\approx84.01g/mol \end{gathered}[/tex]Where do the following boxes on the right belong to?
Answers
According to the question, we are to categorize the definitions in the box as elements, compounds, or mixtures.
For ELEMENTS
• Since element,s consist of only one kind of atom,, hence they are ,substances made up of one type of particle (atoms or molecules)
• Since matter is anything that has mass and occupies space and is made up of substances called elements, hence, they are anything that has mass and volume
,• Cannot be broken into simpler kinds, of matter since elements are made of atoms.
For COMPOUNDS
• Compounds can only be separated by chemical means but not by physical means. Hence we can say that ,compounds cannot be separated by physical means, but can be chemically
• Consists of atoms of two or more different elements bonded together
,• Has properties unique to the element that makes it up
FOR MIXTURES
• Since the mixture consists of, two or more different elements ,and/or compounds physically, hence they can be separated by physical means.
• They can be separated into simpler types of matter by physical means and keeps the, properties of parts, that make it up.
When atoms become bonded with covalent bonds, the result is called a(n):A. ionic bond.B. ion.C. valence.D. molecule.SUBMIT
Answers
Explanation:
A covalent bond occurs when electrons are shared by atoms. Atoms will covalently bond with other atoms to gain stability.
A group of atoms held together by covalent bonds is called a molecule.
Answer: D. molecule
Atom A and B both have 10 protons A have 10 B has 11 neutrons, which statement is true A,b have the same element, theyvhave differnet elements, if they have the same mass, only atom b is a isotope
Answers
Atom A and B both have 10 protons A have 10 B has 11 neutrons is A and B are isotopes of same element.
Isotopes are the atoms of same elements , that has same number of proton but different number of neutrons , mass no. is depends on neutrons present in an atom. the given situation is :
number of proton number of neutron
Atom A 10 10
Atom B 10 11
Isotopes are the members of the same family of same elements.
Thus, Atom A and B both have 10 protons A have 10 B has 11 neutrons is A and B are isotopes of same element.
To learn more about Isotopes here
https://brainly.com/question/17335691
#SPJ1
I need help on #5-#7
Answers
If 5750 J of energy are added to 455 g of granite at 24 °C. The final temperature of granite is 39.9 °C
Given that :
heat energy = 5750 J
mass of granite = 455 g
initial temperature = 24 °C
specific heat capacity = 0.12 cal / g °C = 0.790 J/ g °C
the expression for the heat capacity is given as :
Q = mcΔT
5750 J = 455 g × 0.790 J/ g °C ( T - 24 °C)
5750 = 359.45 T - 8626.8
T = 39.9 °C
if 5750 J of energy are added to 455 g of granite at 24 °C. The final temperature of granite is 39.9 °C
To learn more about heat capacity here
https://brainly.com/question/28302909
#SPJ1
C3H7OH + O2 -> CO2 + H20balance the equation then find mole ratio of oxygen to water, how many mores of carbon dioxide are produced when 4.6 mol of oxygen react, and how many molecules of C3H7OH will react with 4.6 L of O2?
Answers
mole ratio = 9:8
moles of CO2 = 3.07moles
molecules of C3H7OH = 2.75 * 10^22 molecules
ExplanationsThe balanced form of the chemical rreaction is as shown below;
[tex]2C_3H_7OH+9O_2\rightarrow6CO_2+8H_2O[/tex]The mole ratio of oxygen to water as shown in the equation is 9:8
Given the following parameter
mole of oxygen that reacted = 4.6moles
According to stoichiometry, 9moles of oxygen produces 6 moles of caron dioxide. The moles of CO2 that is required will be expressed as:
[tex]\begin{gathered} moles\text{ of CO}_2=\frac{6}{9}\times4.6moles\text{ of O}_2 \\ moles\text{ of CO}_2=3.07moles \end{gathered}[/tex]Hence the moles of CO2 that will be produced is 3.07moles
Also if 4.6L of O2 reacted, the moles of oxygen that reacted will be;
[tex]\begin{gathered} moles\text{ of O}_2=\frac{4.6}{22.4}(1mole\text{ = 22.4L}) \\ moles\text{ of O}_2=0.205moles \end{gathered}[/tex]According to stochiometry, 2 moles of C3H7OH reacts with 9moles of oxygen, the moles of C3H7OH required will be:
[tex]\begin{gathered} moles\text{ of C}_3H_7OH=\frac{2}{9}\times0.205 \\ moles\text{ of C}_3H_7OH=0.0456moles \end{gathered}[/tex]Convert the moles of C3H7OH to molecules as shown
[tex]\begin{gathered} molecules\text{ of }C_3H_7OH=0.0456\times6.02\times10^{23} \\ molecules\text{ of }C_3H_7OH=0.275\times10^{23} \\ molecules\text{ of }C_3H_7OH=2.75\times10^{22}molecules \end{gathered}[/tex]To reduce dangerous swelling in the brain, physician sometimes administer intravenous hypertonic saline, a 5.0%(w/v) Solution of salt (NaCI) In water. Calculate the volume of the solution that contains 0.50 g of NaCl. Be sure your answer has unit symbol and his rounded to the correct number of significant digits
Answers
Percent weight/volume (% w/v) is a way of expressing the concentration of a solutuon. The definition of it is:
% w/v = mass of solute /100 ml of solution
We have to find the volume of the 5.0 % (w/v) solution that contains 0.50 g of NaCl. So we are given these values:
Concentration % w/v = 5.0 %
mass of solute = 0.50 g
Since our solution is 5.0 % w/v Nacl, we know that it has 5.0 g NaCl in 100 ml of solution. So if we want to find the volume of solution that contains 0.50 g we can:
0.50 g of NaCl * 100 ml of solution/(5.0 g of NaCl) = 10. ml of solution.
10. mL of solution is the volume that contains 0.50 g of NaCl
can someone help me with this question
Answers
Explanation:
DataP = 1.3 atm
V = 2.1 L
T = 75 °C = 75 + 273 = 348 K
R = 0.0821 atm.L / mol.K
n = ?
SolutionAs, PV = nRT
therefore, n = PV/RT
n = 1.3 x 2.1 / 0.0821 x 348
n = 0.096 mole
A jug of vinegar is 15% (by mass) acetic acid. If a container holds 500 g of this product, how much acetic acid does it contain?
Answers
Answer:
It contains 75g of acetic acid.
Explanation:
With the percent mass (15%) and the mass of the solution of vinegar, we can calculate the amount of acetic acid, using the percent by mass formula:
[tex]\begin{gathered} PercentByMass=\frac{MassOfSolute}{MassOfSolution}*100\% \\ 15\%=\frac{MassOfSolute}{500g}\times100\operatorname{\%} \\ \frac{15\%}{100\operatorname{\%}}*500g=MassOfSolute \\ 75g=MassOfSolute \\ \end{gathered}[/tex]So, it contains 75g of acetic acid.
Oxygen gas is made up of diatomic non-polar covalent molecules. This leads to very weak attraction between molecules. Which statement would this best support?
Answers
The oxygen gas is non polar because there is no interaction between the molecules of the gas.
What is a diatomic molecule?A diatomic molecule is one that has only two atoms in it. These atoms are usually atoms of the same element in the majority of the cases that we encounter. Given the fact that the both atoms belong to the same substance, the electronegativity difference between the atoms is zero.
Given that the electronegativity difference between the atoms is zero, there is little or no interaction between the molecules of the substance. Thus the very weak intermolecular interaction is coming from the fact that there is no kind of interaction between the molecules.
Thus oxygen gas is covalent and the molecules do not interact with each other because the bond is nonpolar.
Learn more about diatomic molecules:https://brainly.com/question/11815815
#SPJ1
Nitrogen and hydrogen react to form ammonia according to the reaction show.
3H2(g) + N2 <- -> 2NH3.
What is the percent yield if 15.68g nitrogen and 10.74 g hydrogen react to form 5.24 g of ammonia?
Answers
The percent yield of the reaction if 15.68 g of nitrogen and 10.74 g of hydrogen react to form 5.24 g of ammonia would be 82.35%.
Stoichiometric problemFrom the balanced equation of the reaction below:
[tex]3H_2(g) + N_2 < - - > 2NH_3.[/tex]
The mole ratio of hydrogen to nitrogen is 3:1.
The molar mass of hydrogen is 2 g/mol
The molar mass of nitrogen is 28 g/mol
Recall that: mole = mass/molar mass
Mole of 10.74 g hydrogen = 10.74/2
= 5.37 mol
Mole of 15.68 nitrogen = 15.68/28
= 0.56 mol
This means hydrogen is in excess while nitrogen is the limiting reactant. The mole ratio of nitrogen and ammonia is 1:2. Thus, the equivalent mole of ammonia produced would be:
0.56 x 2 = 1.12 mol
Molar mass of ammonia = 17 g/mol
Mass of 1.12 mol ammonia = 1.12 x 17
= 19.04 grams
Percent yield of the reaction = 15.68/19.04 x 100
= 82.35%
In other words, the percent yield of the reaction is 82.35%.
More on stoichiometric problems can be found here: https://brainly.com/question/14465605
#SPJ1
CaCO3(s) + 2HCl(aq) ---> CaCl2(s) + CO2(g) + H2O(ℓ)What would be the volume of CO2 (at STP) produced from the complete reaction of 10.0 grams of CaCO3?
Answers
The equation is balanced since the number of atoms of each element is the same on each side of the reaction.
Now, we will calculate the number of moles present in 10 grams of CaCO3. We will use the molar mass of CaCO3 for this
[tex]\begin{gathered} lesofCaCO3=gCaCO_3\times\frac{1molCaCO_3}{MolarMass,\text{gCaCO}_3} \\ MolesofCaCO3=10.0gCaCO_3\times\frac{1molCaCO_3}{100.0869gCaCO_3}=0.1molCaCO_3 \end{gathered}[/tex]Assuming that the rest of the reactants are in excess, 0.1 mol of CaCO3 will react and form 0.1 mol of CO2, since the ratio is 1 to 1.
Now to calculate the volume, we can apply the ideal gas law which tells us:
[tex]PV=nR_{}T[/tex]Where,
P is the pressure (STP) =1 atm
V is the volume in Liters
n is the number of moles = 0.1 mol CO2
R is a constant = 0.08206 (atm L)/(mol K)
T is the temperature (STP)= 273.15K
Now, we clear the volume and write the known values:
[tex]\begin{gathered} V=\frac{nR_{}T}{P} \\ V=\frac{0.1\text{mol}\times0.08206\frac{atm.L}{mol.K}\times273.15K}{1atm} \\ V=2.2L \end{gathered}[/tex]The volume of CO2(at STP) produced would be 2.2L
I’m having trouble figuring out how to do number 13. Iv done some things but still cannot seem to figure it out.
Answers
This is a stoichiometry problem, where we have an initial amount of reactant and we need to find out how much of the product will we end up with, in order to do that we need to:
1. Set up the properly balanced equation, which the question already provided us
2 KClO3 -> 2 KCl + 3 O2
2. Check the molar ratio between the two compounds, which is 2:3, 2 moles of KClO3 will produce 3 moles of O2, but we have 3 moles of KClO3
2 KClO3 = 3 O2
3 KClO3 = x O2
x = 4.5 moles of O2 will be produced from 3 moles of KClO3
3 KClO3
2 KClO3/ 3 O2
4.5 O2
2.Solid iron combines with oxygen gas to form solid iron(III) oxide. Which of the following equations best describes this reaction?Select one:a. Ab. Bc. Cd. D
Answers
ANSWER
option B
EXPLANATION;
The chemical symbol of iron is Fe
The chemical symbol of oxygen is O2
[tex]\text{ 4Fe + 3O}_2\text{ }\rightarrow\text{ 2Fe}_2O_3[/tex]Therefore, the correct answer is B
31.The correct name for the compound that has the formula BaCl2 is...Select one:a. Baride chlorine.b. Barium chloride.c. Barium chlorine.d. Barium chlorite.
Answers
ANSWER
option B
EXPLANATION
Given that;
The molecular formula of the compound is given as BaCl2
The compound contain a metal and a non-metal
Ba means Barium
Cl2 is chloride because its a diatomic gas
Then BaCl2 is called Barium chloride
Therefore, the correct answer is option B
6. What is the percent composition of chlorine in calcium chloride, CaCl₂?a. 32%b. 36%c. 50%d. 64%
Answers
The first step we have to follow is to find the molecular mass of CaCl2.
[tex]\begin{gathered} Ca=40\times1=40 \\ Cl_2=35.45\times2=70.9 \\ CaCl_2=40+70.9=110.9 \end{gathered}[/tex]Finally divide the mass of Cl2 by the mass of CaCl2 and multiply this product times 100:
[tex]\frac{70.9}{110.9}\cdot100=63.93[/tex]It means that the correct answer is 64%.
What is the purpose in the procedure of chemistry lab: Titration?
Answers
1) Titration. This is a common laboratory method used to determine the unknown concentration of a substance.
2) We use a solution with a known concentration. This solution can be an acid (or a base). We fill a burette with this solution.
3) In a flask, we have the solution with unknown concentration and a known volume. It can be a base (or an acid).
4) Let the solution out of the burette and into the flask. We have to do this until the indicator changes color. Read the volume in the burette.
5) Finally, we have to calculate the concentration of the solution.
Perform the following operationand express the answer inscientific notation.5.4x103 x 1.2x107[ ? ]x10[?]Coefficient (green) Exponent (yellow)tCoefficient (green)Enter
Answers
Since the operation is a multiplication, the order in which we made the multiplications doesn't matter, so we can reoprder it:
[tex]5.4\times10^3\times1.2\times10^7=5.4\times1.2\times10^3\times10^7[/tex]Now, we calculate each of them:
[tex]6.48\times10^{3+7}=6.48\times10^{10}[/tex]Need it quickly ples
Answers
Based on the model , the statement best compares the amounts energy in the reactants and the products of photosynthesis is since making sugar uses energy, the combined energy in the bond CO₂ and H₂O is greater than that in the bonds of O₂ and C₆H₁₂O₆
The plants make sugar using the energy that comes from sunlight and it will convert into the carbon dioxide and the water. this is called as photosynthesis. this process occurs in the chloroplast in the plant cell.
Thus, Based on the model , the statement best compares the amounts energy in the reactants and the products of photosynthesis is since making sugar uses energy, the combined energy in the bond CO₂ and H₂O is greater than that in the bonds of O₂ and C₆H₁₂O₆
To learn more about photosynthesis here
https://brainly.com/question/1757345
#SPJ1
What is the pressure in a 7.00 L tank with 1.73 grams of hydrogen gas at 375 K?
Answers
Considering the ideal gas law, the pressure in a 7.00 L tank with 1.73 grams of hydrogen gas at 375 K is 26.5987 atm.
Definition of ideal gas lawAn ideal gas is called a hypothetical or theoretical gas, which would be composed of particles that move randomly and without interacting with each other.
An ideal gas is characterized by three state variables: absolute pressure (P), volume (V), and absolute temperature (T). The relationship between them constitutes the ideal gas law, an equation that relates the three variables if the amount of substance, number of moles n, remains constant and where R is the molar gas constant:
P×V = n×R×T
Pressure in this caseIn this case, you know:
P= ?V= 7 Lmass of hydrogen= 1.73 gmolar mass of hydrogen= 1 g/molen= mass of hydrogen× molar mass of hydrogen= 1.73 g÷ 1 g/mole= 1.73 molesR= 0.082 atmL/molKT= 375 KReplacing in the definition of ideal gas law:
P× 2 L = 1.73 moles ×0.082 atmL/molK ×375 K
Solving:
P= (1.73 moles ×0.082 atmL/molK ×375 K)÷ 2 L
P= 26.5987 atm
Finally, the pressure is 26.5987 atm.
Learn more about ideal gas law:
brainly.com/question/4147359
#SPJ1
Determine the [H+] of a solution that has a [OH-] of 6.25*10-12. (Enter answer in expanded format not scientific notation)
Answers
answer and explanation
we are given the [OH] concentration as 6.25*10-12
and we know that
[H+][OH-] = 1.0*10^-14
an so using this relationship we can determine the [H+]
[H+][6.25*10-12] = 1.0*10-14
[H+] = 0.0016M
How many grams of H3PO4 could be produced from 94 g of H2O and 186 g of PCL5 according to the equation below?
Answers
Answer:
[tex]89.4\text{ g of H}_3PO_4[/tex]Explanation:
Here, we want to get the mass of H3PO4 produced
We start by getting the balanced equation of reaction:
[tex]PCl_5\text{ + 4H}_2O\text{ }\rightarrow\text{ 5HCl + H}_3PO_4[/tex]The mass of H3PO4 produced would be based on the limiting reactant
The limiting reactant here is the one that would produce less amount of the product H3PO4
Firstly, let us get the number of moles of H3PO4 produced by each of the reactants
To get this, we start by getting the number of moles of each that reacted
We get this by dividing the masses by the molar masses
For water, we have it that the molar mass is 18 g/mol
Thus, the number of moles would be:
[tex]\frac{94}{18}\text{ = 5.2 moles}[/tex]From the balanced equation of reaction:
4 moles of water produced 1 mole of H3PO4
5.2 moles of water will produce x moles of H3PO4
Thus:
[tex]\begin{gathered} x\text{ }\times4\text{ = 5.2}\times1 \\ x\text{ = }\frac{5.2}{4}\text{ = 1.3 mole} \end{gathered}[/tex]For PCl5
The molar mass is 208 g/mol
Thus,we have the number of moles as:
[tex]\frac{186}{208}\text{ = 0.894 mol}[/tex]From the equation of reaction:
1 mol of PCl5 produced 1 mol of H3PO4
That means 0.894 mol of PCl5 produced 0.894 mol of H3PO4
Since PCl5 produced a lesser amount of moles of water, that means, it is the limiting reactant
To get the mass of H3PO4 produced, we multiply this number of moles by
the molar mass of H3PO4
The molar mass of H3PO4 is 100 g/mol
Thus, we have the mass of H3PO4 produced as:
[tex]0.894\text{ }\times\text{ 100 = 89.4 g}[/tex]