Answers
Answer:
a. N3-
b. Ca
c. As
d. Al
Explanation:
a. N or N3-
In this case, the largest species is N3- because it has 3 additional electrons in contrast to the neutral atom.
b. Ca or Ca2+
In this case, the largest species is Ca, because Ca2+ has 2 fewer electrons than the neutral atom of Ca.
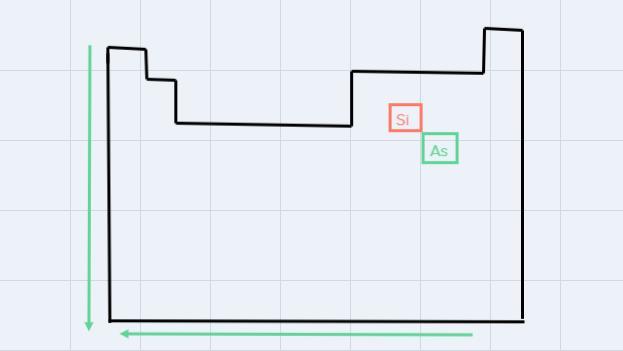
c. Si or As
To analyze these elements, is is necessary to look for them in the Periodic Table of elements:
Since the atomic radius increases from top to bottom and from right to left, the largest is As.
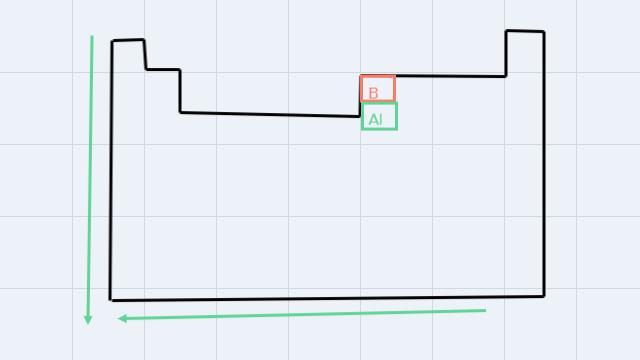
d. Al or B
In this case, we also have to look for the elements in the Periodic table, and following the atomic radius increase, the largest is Al.
Related Questions
How many moles are there for silver that are produced
Answers
0.96moles
Explanations:Given the reaction between Magnessium and silver nitrate expressed as:
[tex]Mg(s)+2AgNO_3(aq)\rightarrow Mg(NO_3)_2(aq)+2Ag(s)[/tex]Given the following parameter
Moles of Magnessium at the start = 0.480moles
According to stoichiometry, 1mole of magnessium produce 2 moles of silver. Hence the moles of silver that is produced if 0.48moles of Mg reacted is given as:
[tex]\begin{gathered} mole\text{ of Ag = 2}\times0.48moles \\ mole\text{ of Ag =}0.96moles \end{gathered}[/tex]Hence the moles of silver that will produced is 0.96moles
Silver nitrate reacts with potassium chloride according as indicated by the balanced equation:AgNO₃ + KCl -----> AgCl + KNO₃How many grams of KCl would be required to react with 380 mL of 0.71 M AgNO₃ solution?.Answer in units of grams
Answers
Silver nitrate reacts with potassium chloride according as indicated by the balanced equation:
AgNO₃ + KCl -----> AgCl + KNO₃
How many grams of KCl would be required to react with 380 mL of 0.71 M AgNO₃ solution?.
First we have to find the number of moles in the solution of AgNO₃, then we can find the number of moles of KCl that will react with those moles of AgNO₃, and finally we can convert the moles of KCl into grams using the molar mass of KCl.
So, molarity is defined as the number of moles of solute divided the volume of solution in L.
Molarity = moles of AgNO₃/volume of solution in L
moles of AgNO₃ = molarity * volume of solution in L
We have 1000 mL in 1 L. We can use that conversion to find the volume of solution in L.
1000 mL = 1 L
Volume of solution in L = 380 mL * 1 L /1000 mL = 0.380 L
Volume of solution in L = 0.380 L
Now we can find the number of moles contained in 380 ml (or 0.380 L) of 0.71 M AgNO₃ solution.
moles of AgNO₃ = molarity * volume of solution in L
moles of AgNO₃ = 0.71 M * 0.380 L
moles of AgNO₃ = 0.270 moles
AgNO₃ + KCl -----> AgCl + KNO₃
We can compare the equation of a chemical reaction with a recipe. The coefficients are the quantities of that recipe. Since all the coefficients are 1, we can read our reaction like this: "1 mol of AgNO₃ will react with 1 mol of KCl to produce 1 mol of AgCl and 1 mol of KNO₃".
The reaction between AgNO₃ and KCl is 1 to 1. We will use that relationship to find the number of moles of KCl that will react with 0.270 moles of AgNO₃.
number of moles of KCl = 0.270 moles of AgNO₃ * 1 mol of KCl/(1 mol of AgNO₃)
number of moles of KCl = 0.270 moles of KCl
Since the reaction is 1 to 1, 0.270 moles of KCl will react with 0.270 moles of AgNO₃.
Finally we can find the mass of KCl. We need the molar mass of KCl.
atomic mass of K = 39.10 amu
atomic mass of Cl = 35.45 amu
molar mass of KCl = 74.55 g/mol
mass of KCl = number of molses of KCl * molar mass of KCl
mass of KCl = 0.270 moles * 74.55 g/mol
mass of KCl = 20.1 g
Answer: 20. g of KCl would be required.
If a block has the following dimensions and a density of 7,8 g/cm what is the masst?
L = 5.0 cm
W = 6.0 cm
H = 2.0 cm
Answers
The question requires us to calculate the mass of a block, given its density (7.8 g/cm~3) and dimensions (length = 5.0 cm; width = 6.0 cm and height = 2.0 cm).
Considering that the volume of the block can be calculated following the basic formula for volume (volume = length x width x height), we can obtain the volume of the object from the dimensions given and then use the definition of density to calculate the mass from the density and volume of the block.
First, we'll calculate the volume of the block:
[tex]\begin{gathered} \text{Volume = length}\times\text{ width }\times\text{ height} \\ \text{Volume = }(5.0cm)\times(6.0cm)\times(2.0cm) \\ \text{Volume = 60 }cm^3 \end{gathered}[/tex]Next, we must consider the definition of density (mass per volume of an object) to calculate the mass of the block:
[tex]\begin{gathered} \text{density = }\frac{mass}{\text{volume}}\to\text{mass = density}\times volume \\ \end{gathered}[/tex][tex]\begin{gathered} \text{mass = (7.8g/}cm^3)\times(60cm^3) \\ \text{mass = }468g \end{gathered}[/tex]Therefore, the mass of the block is 468g.
Which one is not an organic coumpounds
Answers
Answer:
A
Explanation:
There are many definitions, and all of them are organic under some definition. The answer is probably A because it is the only one without hydrogen, and sometimes molecules without hydrogen are counted as being inorganic. A is CCl2F2, which is comprised of two chlorine atoms, two fluorine atoms, and a carbon atom.
An unstable nucleusA-increases its half-lifeB - emits energy when it decaysC- increases its nuclear mass by fissionD- expels all of its protons
Answers
Explanation:
A nucleus consists o both electrons and proton. Therefore, an unstable nucleus undergoes radiation. And this causes a formation of a new atom. This is known as radioactive decay. Therefore, an unstable nucleus emits energy as it decays.
Answer:
The correct answer is B.herefore, an unstable nucleusherefore, an unstable nucleusR
Hi I hope can make a better deal for you and this is a wonderful
Answers
To answer this question we have to find the value of ka, to do it, we can use the value of kb. The product of the kb of a base and the ka of its conjugated base is kw(1x10^-14):
[tex]\begin{gathered} k_a\cdot k_b=k_w \\ k_a=\frac{k_w}{k_b} \\ k_a=\frac{1\times10^{-14}}{4.38\times10^{-5}} \\ k_a=2.88\times10^{-10} \end{gathered}[/tex]Now, we have to use the equation of Hendersson and Hasselbach:
[tex][/tex]The Haber process for producing ammonia commercially is represented by the equation N2(g) + 3H2(g) → 2NH3(g). If 7 L of NH3 are consumed, how many liters of H2 gas are required?
Answers
Step 1
The reaction:
N2(g) + 3H2(g) → 2NH3(g) (balanced and completed)
All gases are assumed to be ideal and to be at STP conditions.
----------------
Step 2
STP conditions:
1 mole of gas = 22.4 L
----------------
Step 3
Information provided:
7 L of NH3 produced (ammonia)
Procedure:
By stoichiometry,
1 mole H2 = 22.4 L
1 mole NH3 = 22.4 L
N2(g) + 3H2(g) → 2NH3(g)
3 x 22.4 L H2 -------- 2 x 22.4 L NH3
X -------- 7 L NH3
X = 7 L NH3 x 3 x 22.4 L H2/2 x 22.4 L NH3 = 10.5 L
Answer: 10.5 L of H2 are consumed.
After being ignited in a Bunsen burner flame, a piece of magnesium ribbon burns brightly, giving off heat and light. In this situation, the Bunsen burner flame providesA)ionization energyB)activation energyC)heat of reactionD)heat of vaporization
Answers
The reaction that involves the combustion of Magnesium will not start unless some amount of energy is added to it, therefore we need the Bunsen burner flame to be the source of the activation energy for the reaction properly occur, since the reaction would not occur in a spontaneous way. Therefore answer letter B
A balloon originally has 0.100 moles of helium and has a volume of 0.500 L. If 0.530 grams of He are added to the balloon, what will the new volume be, in L?
Answers
The new volume of the balloon if 0.530 grams of He are added to the balloon is 1.16 L
What is the number of moles of helium added?The number of moles of helium added is determined as follows:
The final volume of the balloon if 0.20 moles of gas are added will be 1.62 L.
Moles = mass / molar mas
molar mass of helium = 4 g/mol
Moles fo helium added = 0.530 / 4
moles of helium added = 0.1325 moles
The final volume is determined using the ideal gas equation as follows:
PV = nRT
Where;
P is pressureV is volumen is the number of moles of gasR is molar gas constant = 0.082 atm.L/K.molInitial volume, V₁ = 0.50 L
Initial mole of gas, n₁ = 2.50 mol
With pressure and temperature held constant:
Final moles of gas, n₂ = 0.100 + 0.1325
n₂ = 0.2325 mols
Final volume, V₂ = n₂ * V₁ / n₁
V₂ = 0.2325 * 0.5 / 0.1
V₂ = 1.16 L
Learn more about gas volume at: https://brainly.com/question/27100414
#SPJ1
FeCl3 (aq) undergoes a double displacement reaction with Ba(OH)2 (aq). If this reaction produces a solid product containing iron, and the other product is in an aqueous solution, what's the balanced chemical equation for this chemical reaction? Briefly explain how to arrive at the balanced reaction equation.
Answers
The displacement reaction is given as; [tex]2FeCl_{3} + 3Ba(OH)_{2} ----- > 2Fe(OH)_{2} (s) +3 BaCl2[/tex]. We can see that a precipitate was obtained in the reaction.
What is a displacement reaction?We know that a displacement reaction is a reaction that involves the combination of two compounds in which there is a kind of exchange between the reactants. We know in this case that this is a double replacement reaction. The anions that are in the system would exchange partners in the products.
Let us now look at the reaction and try to see how to be able to obtain the reaction. One more time the reaction is going to occur between the aqueous solutions of iron III chloride and barium hydroxide. We must recall that the insoluble product is iron III hydroxide.
We know that the product that is observed is a solid and an aqueous products from the reaction.
Learn ore about displacement reaction:https://brainly.com/question/29307794
#SPJ1
what is the water cycleA process by which water moves from cloud in the skyB A process by which water is absorbed into soil on earth surfaceC A process by which water moves from earth surface into the sky and back againD A process by which wind washes large amounts of water onto land from the oceans
Answers
Since water cycle is a multi-process cycle, the best option that describes and summarize all the cycle, will be letter C, in which we have water moving from the surface of the Earth to the sky and back again as rain, and then back to the sky again as water vapor.
Answer:
(C) A process by which water moves from Earth's surface into the sky and back again
Explanation:
hope this helps! <3
How to draw (E)‑3‑methyl‑2‑pentene
Answers
In (E)‑3‑methyl‑2‑pentene, methyl group is attached in opposite side of the carbon that why it will be name as (E)‑3‑methyl‑2‑pentene.
The name of the given compound is (E)‑3‑methyl‑2‑pentene.
There are 5 carbon atom present in the compound. Methyl group is attached with C-2 and C-3 carbon atom in opposite direction
The structure of (E)‑3‑methyl‑2‑pentene is given as;
In the structure, it can be seen that methyl group is attached in opposite direction.
Trans molecule is named as E -compound.
To know more about Trans molecule
https://brainly.com/question/13981353
#SPJ1
When 1.72 grams of copper metal react with 12.33 grams of silver nitrate how much silver is produced?
Answers
The silver produced is 7.77 g.
Given
Mass of Cu (copper) = 1.72 g.
Mass of AgNO3 (silver nitrate) = 12.33 g.
Molar mass of Cu = 63.5 g/mol.
Molar mass of AgNO3 = 170 g/mol.
Molar mass of Ag = 108 g/mol.
Step-by-step solution:
Cu + 2AgNO₃ --> Cu(NO₃)₂ + 2 Ag
First, let's state the chemical equation with copper (Cu) and silver nitrate (AgNO3) reacting:
1.72 g Cu . [tex]\frac{1 mol Cu}{63.5 g Cu}[/tex] = 0.027 moles of Cu
12.33 g AgNO₃ . [tex]\frac{1 mol AgNO_{3} }{170 g AgNO_{3} }[/tex] = 0.072 moles of Ag
Now, let's find the number of moles of each reactant using their molar mass:
0.027 moles Cu . [tex]\frac{2 moles Ag}{1 mol Cu}[/tex] = 0.054 moles Ag.
Now, let's see how many moles of Ag are being produced based on the number of moles that we found in each reactant. By doing this calculation, we will find the limiting reactant.
You can see in the chemical equation that 1 mol of Cu reacted, produces 2 moles of Ag, so the moles of Ag produced are:
And you can see that moles of AgNO₃ reacts, produces 2 moles of Ag too. This means that the molar ratio between them is 2, more simply sis 1:1. This is telling u that 0.072 moles of AgNO₃ will produce 0.0072 moles of Ag too.
In this case, as you can see the limiting reactant is AgNO₃ because this is being consumed first in the reaction, so the final step is to find the mass using its molar mass. The conversion from 0.0072 moles of Ag to mass in grams is:
0.072 mol Ag . [tex]\frac{108 g Ag}{1 mol Ag}[/tex] = 7.77
The silver that is produced by 0.0072 moles is 7.77 g of Ag (silver).
To learn more about moles, refer: https://brainly.com/question/26416088
#SPJ9
6. What would be the concentration of a solution formed when 1.00 g of NaCl are dissolved in water to make100 mL of solution?
Answers
To find the concentration we can do it in terms of molarity. Molarity is a way of expressing the concentration of a solute in a solution, it is expressed with the term M and can be described by the following equation:
[tex]Molarity=\frac{MolesSolute}{Lsolution}[/tex]So first we must find the moles of NaCl present in 1.00 grams, the moles of NaCl will be:
[tex]\begin{gathered} molNaCl=givengNaCl\times\frac{1molNaCl}{MolarMass,gNaCl} \\ molNaCl=1.00gNaCl\times\frac{1molNaCl}{58.44gNaCl}=0.017molNaCl \end{gathered}[/tex]So, the molarity of the solution will be:
[tex]Molarity=\frac{0.017molNaCl}{0.100Lsolution}=0.17M[/tex]Answer: The concentration of the solution in molarity will be 0.17M
Can you describe the two different kinds of intermolecular forcesthat occur between diol molecules within a liquid?
Answers
A diol molecule is a compound that contains two Hydroxyl groups (OH), and these hydroxyl groups are attached to a hydrocarbon chain, which makes the main chain of the compound. In this type of molecule, we can find two intermolecular forces
Between the Carbons and Hydrogens in the hydrocarbon chain we have a Van der Waals force, which is a weak force
In the Hydroxyl groups, we will have a Hydrogen bond, which will be connecting Hydrogens and Oxygens
Dichlorobenzene exists in three forms. For each of the following write the name of the compound with a numerical designation indicating the location of the chlorine atoms A. Ortho - dichlorobenzene B. Para - dichlorobenzeneC. Meta - diclorobenzine
Answers
Please, look at the next drawing:
Read the water level with the correct number of significant figures.
Answers
Reading significant figures. When you're reading signif
In the following chemical wording, which atom gains electrons?(The formula is in the picture)The answers to choose are:•none•aluminum•oxygen•iron
Answers
Hierro
El número de oxidación del hierro en el lado izquierdo de la reacción es +3 mientras que en el lado derecho está como hierro metálico, es decir su número de oxidación es 0
Hellllp pleaseeeee!!!
Answers
Answer:
A
Explanation:
The molecule is an amino acid which is used to make proteins, so it is an organic molecule.
It has a double bond between c=o
and single bonds throughout the molecule
Hope this helps!
Find the formula mass of the compound, then divide the individual element total by the total mass-move the decimal over two to change it to percentage Al2O3
Answers
1) Find the formula mass of Al2O3.
Aluminum mass: 26.982 u.
Oxygen mass: 15.999 u.
Al2O3 mass = 2 * (26.982 u) + 3 * (15.999 u)
Al2O3 mass = 101.961 u.
2) Aluminum percentage.
Aluminum mass: 26.982 u.
Al2O3 mass = 101.961 u.
[tex]\frac{(2*26.982\text{ }u)}{101.961\text{ }u}=0.5293[/tex]Moving the decimal point.
52.93%
The percentage of aluminum is 52.93%
3) Oxygen percentage.
Oxygen mass: 15.999 u.
Al2O3 mass = 101.961 u.
[tex]\frac{(3*15.999\text{ }u)}{101.961\text{ }u}=0.4707[/tex]Moving the decimal point.
47.07%
The percentage of oxygen is 47.07%.
.
Hello , can I have help with number 43 , please? Can you give explanation for all the choices ? Tank you
Answers
Number 43:
Please look at the next drawing:
Answer: b. upper right
how many significant figures do the following numbers have?1) 5.9 x 104
Answers
Significant figures correspond to the number of digits that a number contains. Zeros at the beginning and end of the number are not counted, only zeros are counted if they are in an intermediate position.
For this case, the number is written in scientific notation, the corresponding 10 of the scientific notation is not taken into account during the digit count, therefore the significant figures of this number will be:
Answer: In 5.9 x10^4 there are 2 significant figures.
Which factors affect electronegativity?
A. Atomic radius and number of unshielded protons
B. Number of electrons and atomic radius
C. Number of unshielded electrons and density of the element
D. Density of the element and atomic radius
Answers
The factors that affect electronegativity is Number of electrons and atomic radius. Option A
What is electronegativity?The term electronegativity has to do with the the bonding situation between two atoms. In this situation, the electrons of the bond would be closer to one of the atoms than the other. In that case, there would be an unequal sharing of the electrons.
There are several facts that affect the electronegativity of an atom and these are the things that determine how electronegative that any particular atom would be.
Learn more about electronegativity:https://brainly.com/question/17762711
#SPJ1
Problem set3. What is the [OH-] of a solution that has a pH of 2?
Answers
We have a solution that pH = 2
We can use this relation which says:
pH + pOH = 14
If we clear pOH from here:
pOH = 14 - pH = 14 - 2 = 12
So, pOH = 12 and pOH could be calculated as:
pOH = - log [OH-] => we clear [OH-] from this,
10^(-pOH) = [OH-] => 10^(-12) = [OH-] => 1x10^-11 mol/L = [OH-]
(mol/L is generally the unit for [OH-])
Answer: [OH-] = 1x10^-11 mol/L
I believe the answer is 4 fold but I'm not sure
Answers
Its volume will be 12-fold.
From the Ideal Gas formula we know that:
[tex]\frac{P_{1\text{ }}\cdot V_1}{T_1}=\frac{P_2\cdot V_2}{T_2}[/tex]- P is the pressure os the gas.
- V is the volume of the gas.
- T is the temperature of the gas.
So, we can replace the hypothetical values to calculate the volume of the gas:
[tex]\begin{gathered} \frac{P_{1\text{ }}\cdot V_1}{T_1}=\frac{P_2\cdot V_2}{T_2} \\ \\ \frac{P_{1\text{ }}\cdot V_1}{T_1}=\frac{P_{1\cdot}\frac{1}{3}\cdot V_2}{T_1\cdot4} \\ V_1=\frac{P_{1\cdot}\frac{1}{3}\cdot V_2\cdot T_1}{P_{1\text{ }}\cdot T_1\cdot4} \\ V_1=\frac{_{}\frac{1}{3}\cdot V_2_{}}{_{}4} \\ V_1\cdot4\cdot3=V_2 \\ V_1\cdot12=V_2 \end{gathered}[/tex]Finally, its volume will be 12-fold.
19.When a compound is added to water only a few of its molecules dissociate to produce hydrogen ions. It is a...Select one:a. strong acid.b. weak acid.c. strong base.d. weak base.
Answers
ANSWER
Weak acid ------ option B
EXPLANATION
Acid is divided in strong and weak acid
Generally, acid can be defined as a substance that will produce hydrogen ions as the only positive ions when dissolved in water.
Strong acid will dissolved completely in water to give hydrogen ions. Example of a strong acid is Hydrogen
Weak acid will dissolved partially in water to give hydrogen ions
Hence, a compound that will give only few of its molecules to produce hydrogen ions when dissolved in water is called a weak acid
Therefore, the correct answer is weak acid
Determine the following elements using their quantum #s of the elements last placed e-.include the e- configuration of the last placed electron.A. n = 4 l = 1, m = +1 , s = - ½,
Answers
If the question is asking us to find the element based on the quantum numbers of the last electron, we have these informations:
n = 4
l = 1
m = +1
s = -1/2
n represents the shell or level value, this value will generally go from 1 to 7, and since our value is 4, we are talking about an element whose valence shell is 4, so this element is in the 4th period
l represents the value of the orbital, this value can range from 0 to 3,
0 is the s orbital
1 is the p orbital
2 the d orbital
3 = f orbital
In out question we have l = 1, therefore the last electron of this element is in the p orbital and in the 4th period, now we are down to 6 elements, Gallium, Germanium, Arsenic, Selenium, Bromine and Krypton, all 6 elements are in the 4th period and have the p orbital as its last one
m represents the location of the electron within the orbitals, each orbital can hold 2 electrons only, p orbitals can hold 6 electrons in 3 pairs of 2, and the m value represents in which orbital the electron is, for l = 1, m can be -1, 0, +1
and in our question we have m = +1, therefore the electron is located in the last orbital, it can only be the 3rd electron or the 6th electron now, and now we are down to Arsenic and Krypton
s is the spin value of the electron, this value will tell us if the electron is pointing upwards or downwards in the orbital, this value can only be +1/2 (upwards) and -1/2 (downwards), and in our question, we have -1/2, which is the last electron of the orbital
Based on all that information, we conclude that this element is Krypton, with 36 of atomic number and electron configuration of [Ar]3d10 4s2 4p6
15. NI3 decomposes into Nitrogen gas and Iodine. If you start with 0.02 grams of NI3,How many moles of Nitrogen are produced at STP?
Answers
0.0000253moles
Explanations:The decomposition of NI3 is given as shown below;
[tex]2NI_3(s)→N_2(g)+3I_2(g)[/tex]Given the following parameters
Mass of NI3 = 0.02 grams
Determine the moles of NI3
[tex]\begin{gathered} moles\text{ of NI}_3=\frac{mass}{molar\text{ mass}} \\ moles\text{ of NI}_3=\frac{0.02}{394.719} \\ moles\text{ of NI}_3=0.0000507moles \end{gathered}[/tex]Acoording to stochiometry, 2 moles of NI3 preoduces 1 mole of nitrogen. The mole of nitrogen produced is;
[tex]\begin{gathered} mole\text{ of nitrogen}=\frac{1}{2}\times0.0000597 \\ mole\text{ of nitrogen}=\text{0.0000253moles} \end{gathered}[/tex]Hence the moles of nitrogen produced is 0.0000253moles
the following unbalanced equation is performed in a laboratory:Mg + HCl --> MgClz + H2If you begin with 10.0 grams of each reactant, how many grams of hydrogen gas should you expect to measure in your experiment?
Answers
Answer: 0.276g of H2 could be obtained from the reaction, considering the mass of reactants given
Explanation:
The question requires us to determine the amount of hydrogen gas (H2) that would be obtained when 10.0g of metallic magnesium (Mg) and 10.0g of chloridric acid (HCl) are reacted.
The following unbalanced chemical equation was provided:
[tex]Mg+HCl\rightarrow MgCl_2+H_2[/tex]To solve this problem, we'll need:
1) obtain the balanced chemical equation;
2) determine the limiting reactant considering the amount of reactants used and the stoichiometry of the reaction;
3) calculate the mass of hydrogen gas produced, considering the limiting reactant
1) Balancing the chemical equation
From the unbalaced chemical equation, we can see that there is the same amount of Mg on both sides of the equation, but we need to adjust the amount of H and Cl atoms.
There are 2 Cl atoms and 2 H atoms on the right side, and 1 Cl and 1 H atom on the left side, thus we can adjust the coefficient of HCl from 1 to 2 to adjust these elements. The balanced chemical equation can be written as:
[tex]Mg+2HCl\rightarrow MgCl_2+H_2[/tex]2) Determining the limiting reactant
Now that we know the balanced chemical equation, we can say that 2 moles of HCl are necessary to react with 1 mol of Mg. Thus, we can write:
1 mol Mg ----------------------- 2 mol HCl
From this stoichiometric relation, we can calculate the amount of HCl that would be necessary to react with 10.0 g of Mg.
First, let's determine the amount of moles of Mg contained in 10.0g of this metal and the number of moles of HCl in 10.0g of this acid:
[tex]\begin{gathered} number\text{ of moles = }\frac{mass\text{ of sample \lparen g\rparen}}{molar\text{ mass \lparen g/mol\rparen}} \\ \\ number\text{ of moles of Mg = }\frac{10.0g}{24.31g/mol}=0.411mol\text{ of Mg} \\ \\ number\text{ of moles of HCl = }\frac{10.0g}{36.46g/mol}=0.274mol\text{ of HCl} \end{gathered}[/tex](the atomic mass of Mg is 24.31 amu, which is numerically identical to its molar mass, 24.31 g/mol, and the molar mass of HCl is 36.4g g/mol)
Therefore, 0.411 moles of Mg and 0.274 moles of HCl were used in the reaction.
Next, we can determine how many moles of HCl would be necessary to react with 0.411 moles of Mg:
1 mol Mg ----------------------- 2 mol HCl
0.411 mol Mg ----------------- x
Solving for x, we have that 0.822 moles of HCl would be necessary to react with the given amount of Mg. Since the amount actually used of HCl (0.274 mol) is smaller than the necessary amount, we can say that HCl is the limiting reactant.
3) Calculating the mass of H2 obtained
Using the limiting reactant and the stoichiometry of the reaction, we can determine the amount of H2 that could be produced.
From the balanced chemical equation, we know that 2 moles of HCl are necessary to produce 1 mol of H2. Thus, we can write:
2 mol HCl ------------------- 1 mol H2
0.274 mol HCl ------------ y
Solving for y, we have that 0.137 moles of H2 would be obtained.
We can convert the number of moles of H2 to its correspondent mass as it follows (the molar mass of H2 is 2.016 g/mol):
[tex]\begin{gathered} number\text{ of moles = }\frac{mass\text{ of sample \lparen g\rparen}}{molar\text{ mass \lparen g/mol\rparen}}\rightarrow mass\text{ of sample = number of moles}\times molar\text{ mass} \\ \\ mass\text{ of H}_2=0.137mol\times2.016g/mol=0.276g \end{gathered}[/tex]Therefore, 0.276g of H2 could be obtained from the reaction, considering the mass of reactants given.
Accessibility ModePrintFindPart II: Limiting Reactant1. Consider the reaction: 5C +2SO₂ → CS₂ + 4COa.) If you had 10 mol of Carbon, how many moles of carbon monoxide would be produced?b.) If you had 10 mol of sulfur dioxide, how many moles of carbon monoxide would beproduced?c.) If you had 10 mol of C and SO2 which reactant would be limiting?d.) What is the theoretical yield of CO, in moles if you react 10 moles of each reactant?SAMEFaPmenSavarہےEP Immersive Reader
Answers
Answer:
a.) 8 moles of carbon monoxide (CO).
b.) 20 moles of carbon monoxide (CO)
c.) C is the limiting reactant.
d.) The theoretical yield of CO would be 8 moles.
Explanation:
Let's write the chemical equation:
[tex]5C+2SO_2\rightarrow CS_2+4CO.[/tex]a.) You can see that 5 moles of carbon (C) reacted produces 4 moles of carbon monoxide (CO), so if we want to know how many moles of carbon monoxide can be produced, we can multiply each coefficient in the chemical equation by 2 (because we will have 10 moles of carbon):
[tex]10C+4SO_2\operatorname{\rightarrow}2CS_2+8CO.[/tex]The answer is that we will produce 8 moles of carbon monoxide (CO) by 10 moles of carbon (C).
b.) We can apply the same logic to this case. In the chemical equation, we have 2 moles of sulfur dioxide (SO2) reacted that produces 4 moles of carbon monoxide (CO), so if we want to know how many moles of carbon monoxide can be produced, we can multiply each coefficient by 5 so we will have 10 moles of sulfur dioxide:
[tex]25C+10SO_2\operatorname{\rightarrow}5CS_2+20CO.[/tex]The answer is that we will produce 20 moles of carbon monoxide (CO) by 10 moles of sulfur dioxide (SO2).
c.) We have already known that 10 moles of C will produce 8 moles of CO, and 10 moles of SO2 will produce 20 moles of CO. If we react 10 moles of C and 10 moles of SO2, you can note that we're going to have an excess of SO2 because we don't have enough amount of C to produce 20 moles of CO as SO2 do. So based on this logic, C is being consumed first and C would be the limiting reactant.
d.) Remember that the theoretical yield indicates the amount of a product obtained in a chemical reaction.
As we saw before, C is the limiting reactant if we react 10 moles of each reactant, so we have already known that 10 moles of C reacted produces 8 moles of CO, so the theoretical yield of CO would be 8 moles.